Organic Research
Research in organic chemistry at UNC Chapel Hill covers a broad range of subjects from synthetic methods development to the design of materials with functions inspired by biological systems. Research is often interdisciplinary and involves science at the frontiers of polymer chemistry, inorganic chemistry, physical chemistry, materials science and bioorganic chemistry.
With nine faculty members formally in the organic division and a number of faculty members from other divisions whose research involves organic chemistry, graduate students find they have a wide range of choices for their doctoral research.
- Synthetic Methods Development
- Natural Products Synthesis
- Chemical Catalysis
- Bioorganic Chemistry
- Molecular Photochemistry
- Supramolecular Chemistry
- Molecular Recognition
- Chemical Biology
- Medicinal Chemistry
- Polymer Synthesis and Functionalization
Recent Research Results
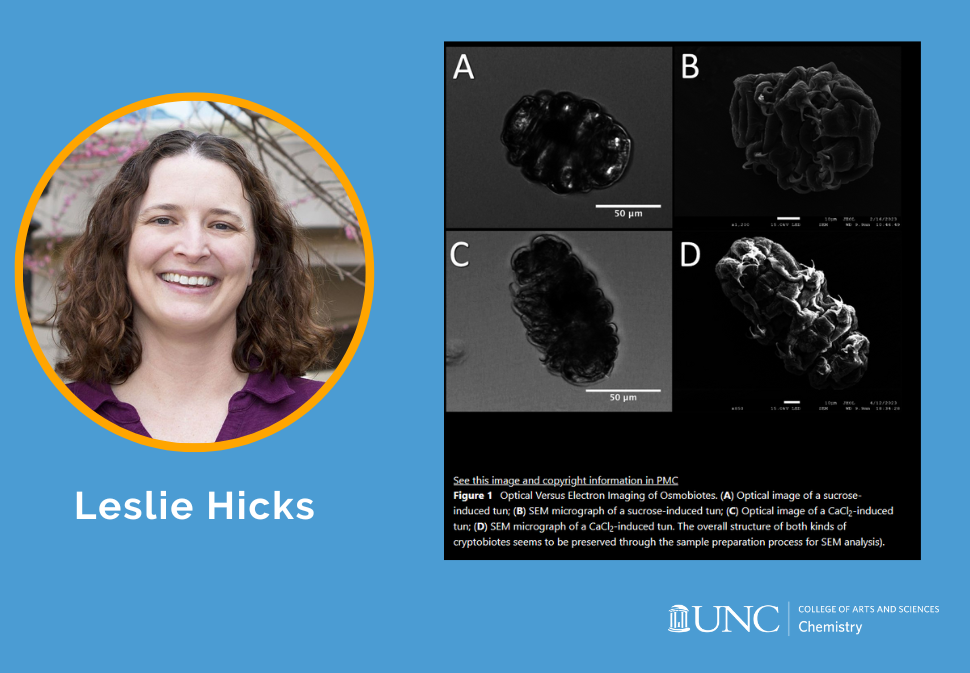
Herein, an approach for discriminating between tardigrade morphological states is developed and utilized to compare sucrose- and CaCl2-induced tuns, using the model species Hypsibius exemplaris.
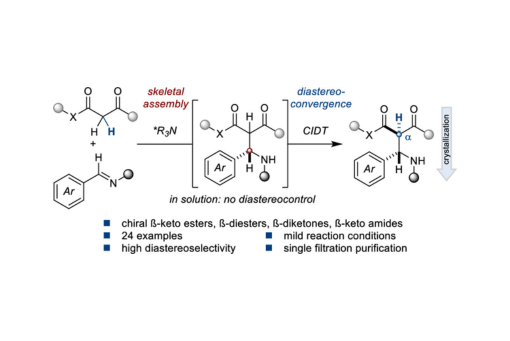
Herein, we disclose a simple catalytic crystallization-driven enantio- and diastereoselective Mannich reaction for the synthesis of stereodefined α-monosubstituted-ß-keto esters, dissymmetric ß-diesters, dissymmetric ß-diketones, and ß-keto amides that productively leverages product epimerization in solution.