Department News

Kevin Weeks, Distinguished Professor of Chemistry at UNC-Chapel Hill, has been named a Senior Member of the National Academy of Inventors.
Gary Pielak has received a Distinguished Scholar Fellowship from the Fulbright U.S. Scholar Program that will fund a four-month research residency in Israel.

In the study, “Evaluating Legacy and Emerging PFAS in Human Blood Collected from 2003 to 2021,” the UNC-led research team wanted to see whether PFAS exposure might be linked to autoimmunediseases.

The UNC-Chapel Hill Department of Chemistry is pleased to share that Megan Jackson, Assistant Professor of Chemistry, has been selected as a 2026 Cottrell Scholar by the Research Corporation for Science Advancement (RCSA).
Research

Herein, an approach for discriminating between tardigrade morphological states is developed and utilized to compare sucrose- and CaCl2-induced tuns, using the model species Hypsibius exemplaris.

Herein, we disclose a backbone rearrangement approach to tune the short-chain branching of polymers.
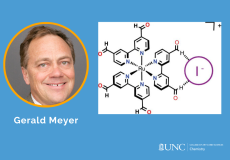
A new homoleptic Ru polypyridyl complex bearing two aldehyde groups on each bipyridine ligand, [Ru(dab)3](PF6)2, where dab is 4,4′-dicarbaldehyde-2,2′-bipyridine, was synthesized, characterized, and utilized for iodide photo-oxidation studies.
Connect