Stereodivergent Nucleophilic Additions to Racemic β-Oxo Acid Derivatives: Fast Addition Outcompetes Stereoconvergence in the Archetypal Configurationally Unstable Electrophile
Abstract
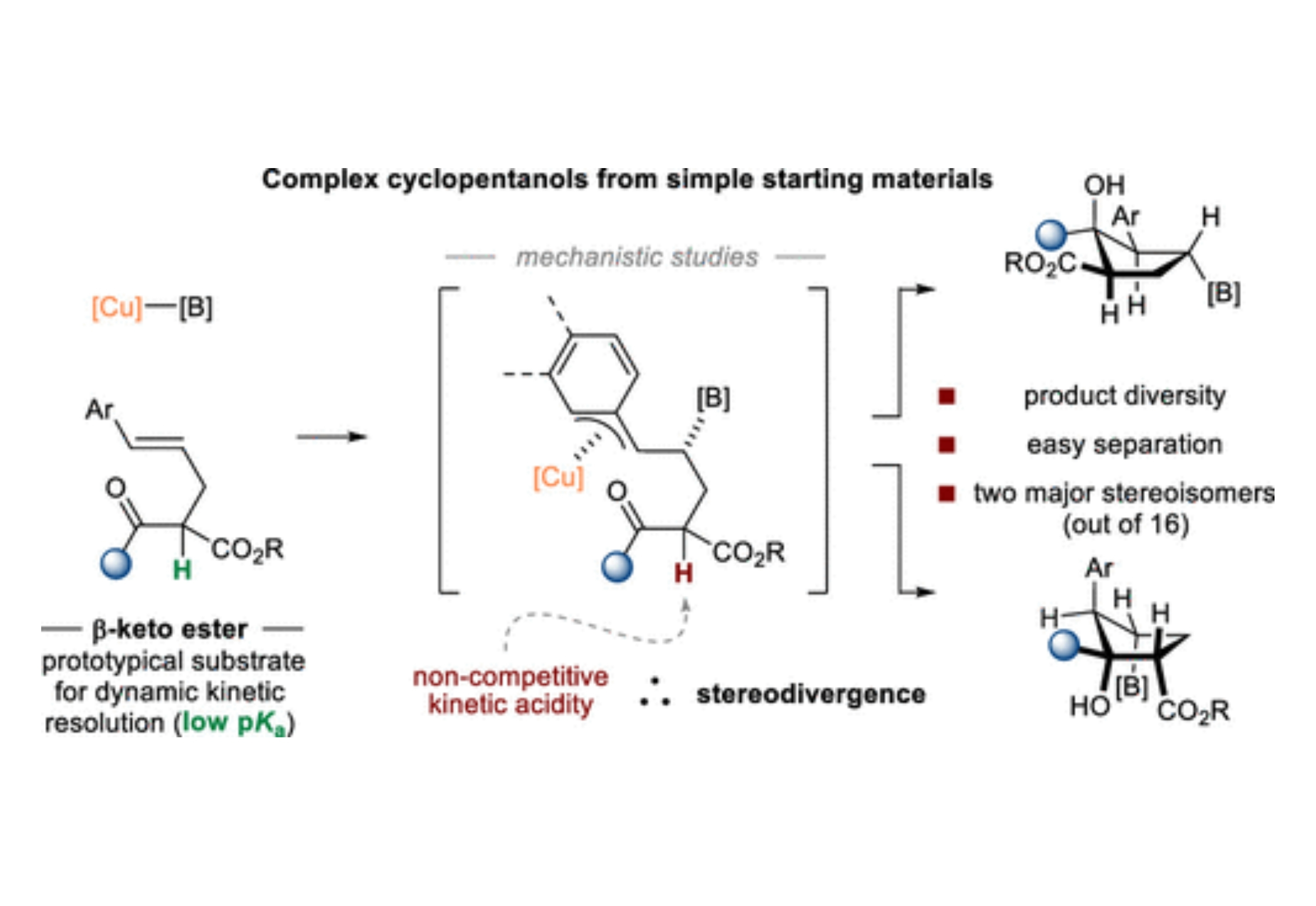
Additions of carbon nucleophiles to racemic α-stereogenic β-oxo acid derivatives that deliver enantiomerically enriched tertiary alcohols are valuable, but uncommon. This article describes stereodivergent Cu-catalyzed borylative cyclizations of racemic β-oxo acid derivatives bearing tethered pro-nucleophilic olefins to deliver highly functionalized cyclopentanols containing four contiguous stereogenic centers. The reported protocol is applicable to a range of β-oxo acid derivatives, and the diastereomeric products are readily isolable by typical chromatographic techniques. α-Stereogenic-β-keto esters are typically thought to have extreme or spontaneous configurational fragility, but mechanistic studies for this system reveal an unusual scenario wherein productive catalysis occurs on the same time scale as background substrate racemization and completely outcompetes on-cycle epimerization, even under the basic conditions of the reaction.
Citation
Stereodivergent Nucleophilic Additions to Racemic β-Oxo Acid Derivatives: Fast Addition Outcompetes Stereoconvergence in the Archetypal Configurationally Unstable Electrophile
Pedro De Jesús Cruz, Evan T. Crawford, Shubin Liu, and Jeffrey S. Johnson
Journal of the American Chemical Society 2021 143 (39), 16264-16273
DOI: 10.1021/jacs.1c07702