Resolving Halide Ion Stabilization through Kinetically Competitive Electron Transfers
Abstract
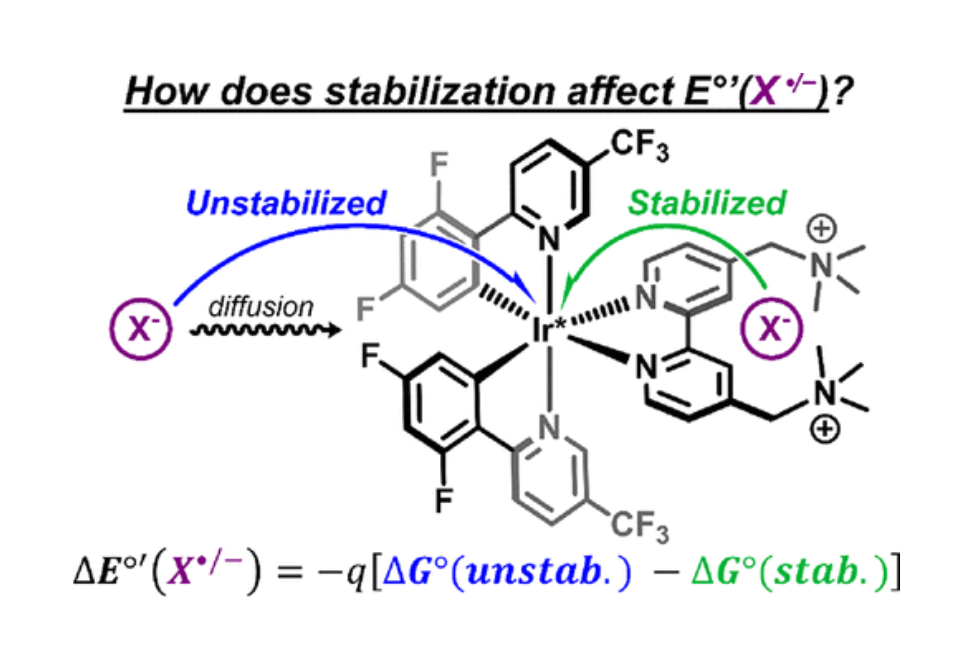
Stabilization of ions and radicals often determines reaction kinetics and thermodynamics, but experimental determination of the stabilization magnitude remains difficult, especially when the species is short-lived. Herein, a competitive kinetic approach to quantify the stabilization of a halide ion toward oxidation imparted by specific stabilizing groups relative to a solvated halide ion is reported. This approach provides the increase in the formal reduction potential, ΔE°′(Χ•/–), where X = Br and I, that results from the noncovalent interaction with stabilizing groups. The [Ir(dF-(CF3)-ppy)2(tmam)]3+ photocatalyst features a dicationic ligand tmam [4,4′-bis[(trimethylamino)methyl]-2,2′-bipyridine]2+ that is shown by 1H NMR spectroscopy to associate a single halide ion, Keq = 7 × 104 M–1 (Br–) and Keq = 1 × 104 M–1 (I–). Light excitation of the photocatalyst in halide-containing acetonitrile solutions results in competitive quenching by the stabilized halide and the more easily oxidized diffusing halide ion. Marcus theory is used to relate the rate constants to the electron-transfer driving forces for oxidation of the stabilized and unstabilized halide, the difference of which provides the increase in reduction potentials of ΔE°′(Br•/–) = 150 ± 24 meV and ΔE°′(I•/–) = 67 ± 13 meV. The data reveal that Keq is a poor indicator of these reduction potential shifts. Furthermore, the historic and widely used assumption that Coulombic interactions alone are responsible for stabilization must be reconsidered, at least for polarizable halogens.
Citation
Resolving Halide Ion Stabilization through Kinetically Competitive Electron Transfers
DOI: 10.1021/jacsau.2c00088