Properties of a tardigrade desiccation-tolerance protein aerogel
Abstract
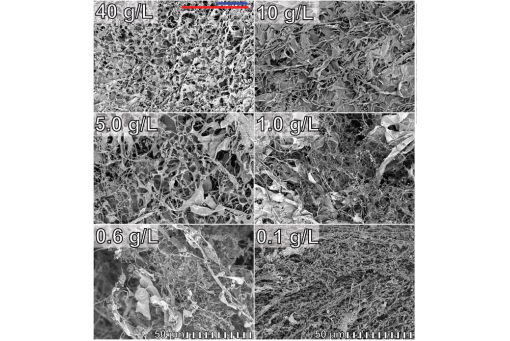
Lyophilization is promising for tackling degradation during the drying and storage of protein-based drugs. Tardigrade cytosolically abundant heat soluble (CAHS) proteins are necessary and sufficient for desiccation-tolerance in vivo and protein protection in vitro. Hydrated CAHS proteins form coiled-coil-based fine-stranded, cold-setting hydrogels, but the dried protein remains largely uncharacterized. Here, we show that dried CAHS D gels (i.e., aerogels) retain the structural units of their hydrogels, but the details depend on prelyophilization CAHS concentrations. Low concentration samples (<10 g/L) form thin (<0.2 μm) tangled fibrils lacking regular structure on the micron scale. Upon increasing the concentration, the fibers thicken and form slabs comprising the walls of the aerogel pores. These changes in morphology are associated with a loss in disorder and an increase in large β sheets and a decrease in α helices and random coils. This disorder-to-order transition is also seen in hydrated gels as a function of concentration. These results suggest a mechanism for pore formation and indicate that using CAHS proteins as excipients will require attention to initial conditions because the starting concentration impacts the lyophilized product.
Citation
Eicher, J., Hutcheson, B. O., & Pielak, G. J. (2023). Properties of a tardigrade desiccation-tolerance protein aerogel. Biophysical Journal, 122(12), 2500–2505. https://doi.org/10.1016/j.bpj.2023.05.002