Photochemical H2 Evolution from Bis(diphosphine)nickel Hydrides Enables Low-Overpotential Electrocatalysis
Abstract
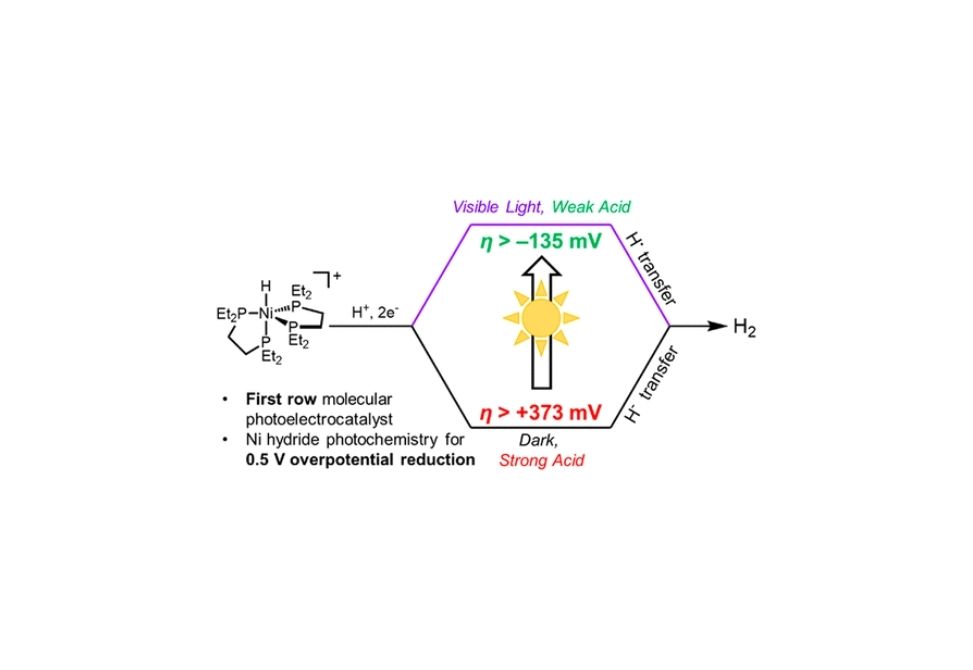
Molecules capable of both harvesting light and forming new chemical bonds hold promise for applications in the generation of solar fuels, but such first-row transition metal photoelectrocatalysts are lacking. Here we report nickel photoelectrocatalysts for H2 evolution, leveraging visible-light-driven photochemical H2 evolution from bis(diphosphine)nickel hydride complexes. A suite of experimental and theoretical analyses, including time-resolved spectroscopy and continuous irradiation quantum yield measurements, led to a proposed mechanism of H2 evolution involving a short-lived singlet excited state that undergoes homolysis of the Ni–H bond. Thermodynamic analyses provide a basis for understanding and predicting the observed photoelectrocatalytic H2 evolution by a 3d transition metal based catalyst. Of particular note is the dramatic change in the electrochemical overpotential: in the dark, the nickel complexes require strong acids and therefore high overpotentials for electrocatalysis; but under illumination, the use of weaker acids at the same applied potential results in a more than 500 mV improvement in electrochemical overpotential. New insight into first-row transition metal hydride photochemistry thus enables photoelectrocatalytic H2 evolution without electrochemical overpotential (at the thermodynamic potential or 0 mV overpotential). This catalyst system does not require sacrificial chemical reductants or light-harvesting semiconductor materials and produces H2 at rates similar to molecular catalysts attached to silicon.
Citation
Photochemical H2 Evolution from Bis(diphosphine)nickel Hydrides Enables Low-Overpotential Electrocatalysis
Bethany M. Stratakes, Kaylee A. Wells, Daniel A. Kurtz, Felix N. Castellano, and Alexander J. M. Miller
Journal of the American Chemical Society 2021 143 (50), 21388-21401
DOI: 10.1021/jacs.1c10628