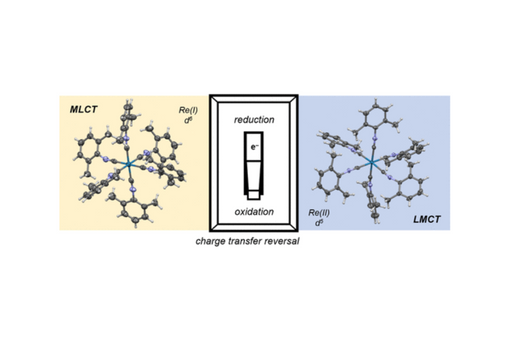
Isocyanide Ligands Promote Ligand-to-Metal Charge Transfer Excited States in a Rhenium(II) Complex
Abstract
A metal-to-ligand charge transfer with mixed intraligand character is observed for the rhenium hexakisarylisocyanide complex [Re(CNAr)6]PF6 (CNAr = 2,6-dimethylphenylisocyanide, λmax = 300 nm). Upon oxidation to [Re(CNAr)6](PF6)2, the dominant low energy optical transition is a ligand-to-metal charge transfer (LMCT) mixed with intraligand transitions (λmax = 650 nm). TD-DFT was used to identify the participating ligand-based orbitals in the LMCT transition, revealing that the majority of the donor orbital is based on the aryl ring (85%) as opposed to the CN bond (14%). For both [Re(CNAr)6]+ and [Re(CNAr)6]2+, structural characterization by X-ray diffraction reveals deviations from Oh geometry at the central Re ion, with larger reduction in symmetry observed for Re(II). For [Re(CNAr)6]+, these structural changes lead to a broadening of the strong ν(C≡N) stretch (2065 cm–1), as the degeneracy of the T1u IR-active mode is broken. Furthermore, a shoulder is observed for this ν(C≡N) stretch, resulting from deviation of the C–N–Ar bond from linearity. By contrast, [Re(CNAr)6]2+ has two weak bands in the ν(C≡N) region (2065 and 2121 cm–1). DFT calculations indicate that reduction of symmetry at the central rhenium ion manifests in the decrease in intensity and the observed split of the ν(C≡N) band. Stability of both complexes are limited by light-induced decomposition where Re(I) dissociates a isocyanide ligand upon irradiation and Re(II) absorbance decays under ambient light. These data provide new insights to the electronic structure of [Re(CNAr)6]2+, enhancing our understanding of LMCT excited states and the versatility of isocyanide ligands.
Citation
Isocyanide Ligands Promote Ligand-to-Metal Charge Transfer Excited States in a Rhenium(II) Complex
Tayliz M. Rodriguez, Mawuli Deegbey, Chun-Hsing Chen, Elena Jakubikova, and Jillian L. Dempsey
Inorganic Chemistry Article ASAP
DOI: 10.1021/acs.inorgchem.2c03193