β-Functionalization of Saturated Aza-Heterocycles Enabled by Organic Photoredox Catalysis
Abstract
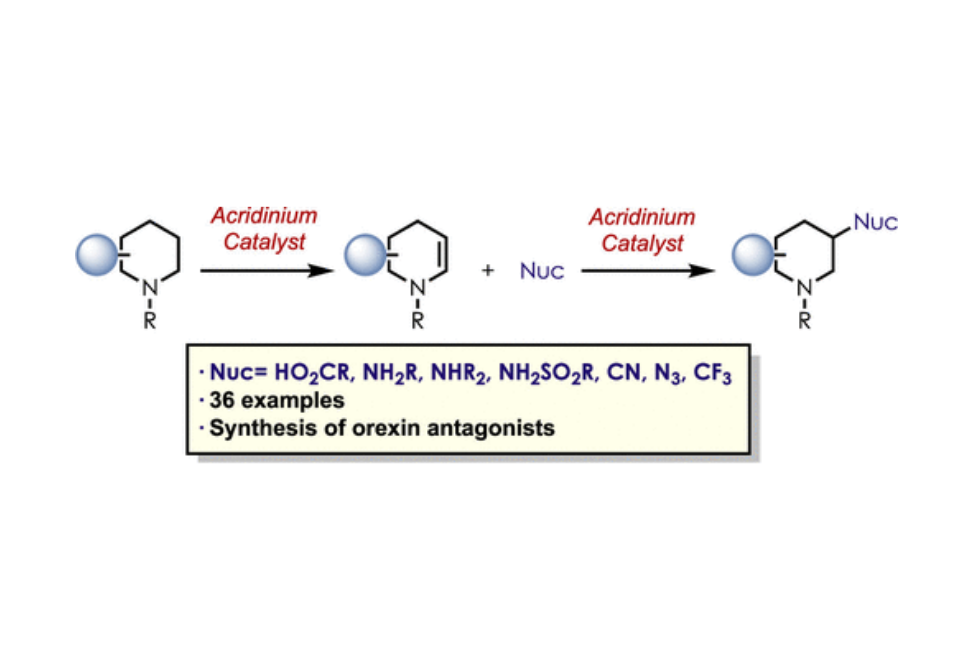
The direct β-functionalization of saturated aza-heterocycles has remained a synthetic challenge because of the remote and unactivated nature of β-C–H bonds in these motifs. Herein, we demonstrate the β-functionalization of saturated aza-heterocycles enabled by a two-step organic photoredox catalysis approach. Initially, a photoredox-catalyzed copper-mediated dehydrogenation of saturated aza-heterocycles produces ene-carbamates. This is followed by an anti-Markovnikov hydrofunctionalization of the ene-carbamates with a range of heteroatom-containing nucleophiles furnishing an array of C–C, C–O, and C–N aza-heterocycles at the β-position.
Citation
β-Functionalization of Saturated Aza-Heterocycles Enabled by Organic Photoredox Catalysis
Natalie Holmberg-Douglas, Younggi Choi, Brian Aquila, Hoan Huynh, and David A. Nicewicz
ACS Catalysis 2021 11 (5), 3153-3158
DOI: 10.1021/acscatal.1c00099