Water’s Variable Role in Protein Stability Uncovered by Liquid-Observed Vapor Exchange NMR
Abstract
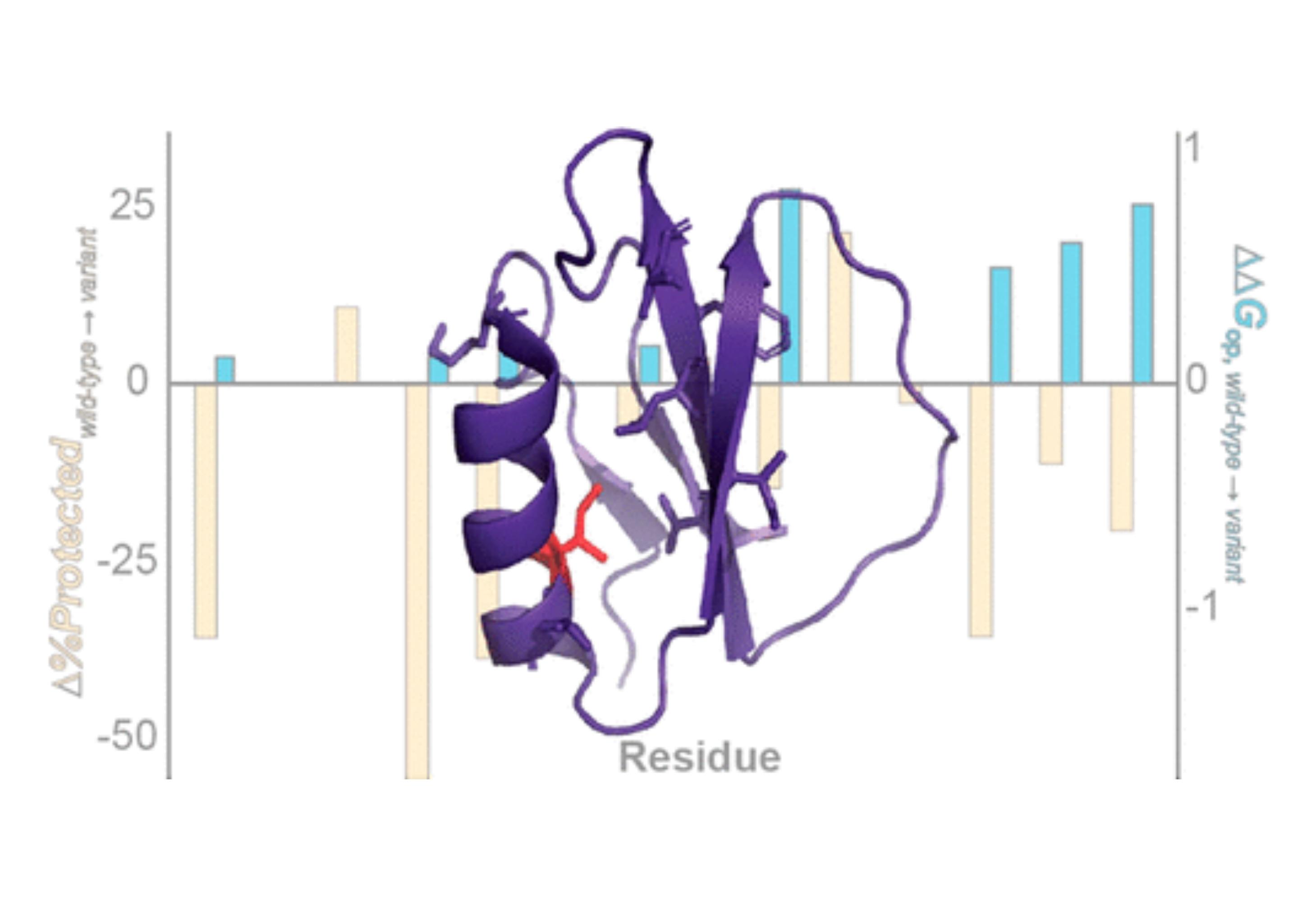
Water is essential to protein structure and stability, yet our understanding of how water shapes proteins is far from thorough. Our incomplete knowledge of protein–water interactions is due in part to a long-standing technological inability to assess experimentally how water removal impacts local protein structure. It is now possible to obtain residue-level information on dehydrated protein structures via liquid-observed vapor exchange (LOVE) NMR, a solution NMR technique that quantifies the extent of hydrogen–deuterium exchange between unprotected amide protons of a dehydrated protein and D2O vapor. Here, we apply LOVE NMR, Fourier transform infrared spectroscopy, and solution hydrogen–deuterium exchange to globular proteins GB1, CI2, and two variants thereof to link mutation-induced changes in the dehydrated protein structure to changes in solution structure and stability. We find that a mutation that destabilizes GB1 in solution does not affect its dehydrated structure, whereas a mutation that stabilizes CI2 in solution makes several regions of the protein more susceptible to dehydration-induced unfolding, suggesting that water is primarily responsible for the destabilization of the GB1 variant but plays a stabilizing role in the CI2 variant. Our results indicate that changes in dehydrated protein structure cannot be predicted from changes in solution stability alone and demonstrate the ability of LOVE NMR to uncover the variable role of water in protein stability. Further application of LOVE NMR to other proteins and their variants will improve the ability to predict and modulate protein structure and stability in both the hydrated and dehydrated states for applications in medicine and biotechnology.
Citation
Water’s Variable Role in Protein Stability Uncovered by Liquid-Observed Vapor Exchange NMR
Candice J. Crilly, Jonathan E. Eicher, Owen Warmuth, Joanna M. Atkin, and Gary J. Pielak
Biochemistry 2021 60 (41), 3041-3045
DOI: 10.1021/acs.biochem.1c00552