Versatile Triphenylphosphine-Containing Polymeric Catalysts and Elucidation of Structure–Function Relationships
Abstract
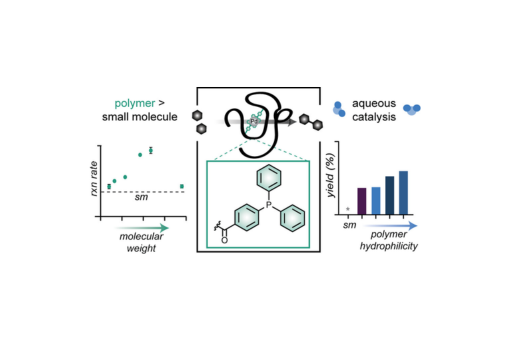
Synthetic polymers are a modular solution to bridging the two most common classes of catalysts: proteins and small molecules. Polymers offer the synthetic versatility of small-molecule catalysts while simultaneously having the ability to construct microenvironments mimicking those of natural proteins. We synthesized a panel of polymeric catalysts containing a novel triphenylphosphine acrylamide monomer and investigated how their properties impact the rate of a model Suzuki–Miyaura cross-coupling reaction. Systematic variation of polymer properties, such as the molecular weight, functional density, and comonomer identity, led to tunable reaction rates and solvent compatibility, including full conversion in an aqueous medium. Studies with bulkier substrates revealed connections between polymer parameters and reaction conditions that were further elucidated with a regression analysis. Some connections were substrate-specific, highlighting the value of the rapidly tunable polymer catalyst. Collectively, these results aid in building structure–function relationships to guide the development of polymer catalysts with tunable substrates and environmental compatibility.
Citation
Versatile Triphenylphosphine-Containing Polymeric Catalysts and Elucidation of Structure–Function Relationships
Matthew A. Sanders, Supraja S. Chittari, Nicole Sherman, Jack R. Foley, and Abigail S. Knight
Journal of the American Chemical Society Article ASAP
DOI: 10.1021/jacs.3c01092