Ultrasensitive Electrochemistry by Radical Annihilation Amplification in a Solid–Liquid Microgap
Abstract
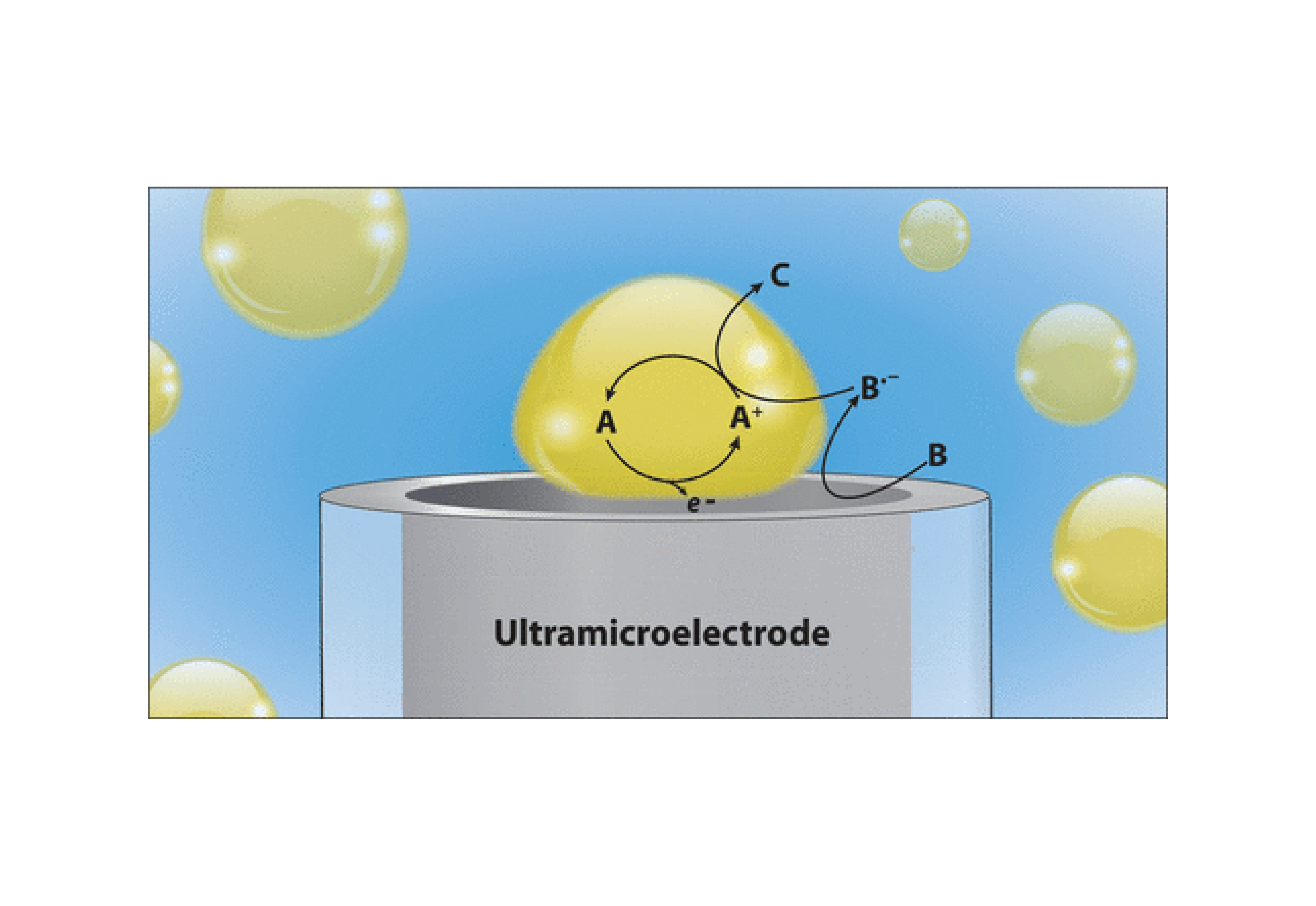
We report a technique to amplify the electrochemical signal within micro- and nanodroplets via radical annihilation amplification. Toluene droplets filled with decamethylferrocene (DmFc) are suspended in an aqueous solution containing 10 mM NaClO4 and 10 μM Na2C2O4. When a toluene droplet irreversibly collides with an ultramicroelectrode biased sufficiently positive for concurrent oxidation of DmFc and oxalate (C2O42–), blip-type responses are observed in the amperometric i-t trace even when the concentration of DmFc is 50 nM. The toluene droplet wetting the ultramicroelectrode effectively creates a microgap, where DmFc molecules are oxidized to DmFc+. In the continuous phase, the oxidation of oxalate (C2O42–) produces a strong reducing agent, CO2•–. Regeneration of DmFc via radical annihilation amplifies the current, similar to conventional nanogap experiments. This experiment allows one to observe the electrochemistry of hundreds to thousands of molecules trapped in a femtoliter droplet, enhancing the sensitivity of droplet-based electrochemistry by 5 orders of magnitude. Finite element simulations validate our experimental results and indicate the importance of the droplet geometry to amplification.
Citation
Ultrasensitive Electrochemistry by Radical Annihilation Amplification in a Solid–Liquid Microgap
Rezvan Kazemi, Nicole E. Tarolla, and Jeffrey E. Dick
Analytical Chemistry 2020 92 (24), 16260-16266
DOI: 10.1021/acs.analchem.0c04183