Tunable Anion Exchange Membrane Conductivity and Permselectivity via Non-Covalent, Hydrogen Bond Cross-Linking
Abstract
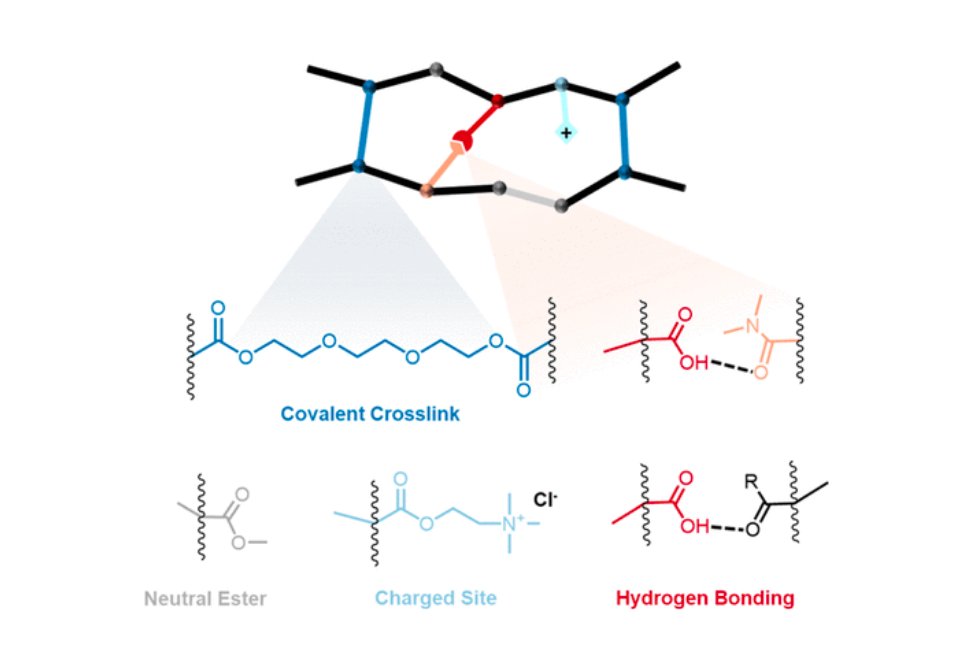
Ion exchange membranes (IEMs) are a key component of electro-chemical processes that purify water, generate clean energy, and treat waste. Most conventional polymer IEMs are covalently cross-linked, which results in a challenging tradeoff relationship between two desirable properties.high permselectivity and high conductivity.in which one property cannot be changed without negatively affecting the other. In an attempt to overcome this limitation, in this work we synthesized a series of anion exchange membranes containing non-covalent cross-links formed by a hydrogen bond donor (methacrylic acid) and a hydrogen bond acceptor (dimethylacrylamide). We show that these monomers act synergistically to improve both membrane permselectivity and conductivity relative to a control membrane without non-covalent cross-links. Furthermore, we show that the hydrogen bond donor and acceptor loading can be used to tune permselectivity and conductivity relatively independently of one another, escaping the tradeoff observed in conventional membranes.
Citation
Tunable Anion Exchange Membrane Conductivity and Permselectivity via Non-Covalent, Hydrogen Bond Cross-Linking
Ryan Kingsbury, Maruti Hegde, Jingbo Wang, Ahmet Kusoglu, Wei You, and Orlando Coronell
ACS Applied Materials & Interfaces 2021 13 (44), 52647-52658
DOI: 10.1021/acsami.1c15474