Switching between X-Pyrano-, X-Furano-, and Anhydro-X-pyranoside Synthesis (X = C, N) under Lewis acid Catalyzed Conditions
Abstract
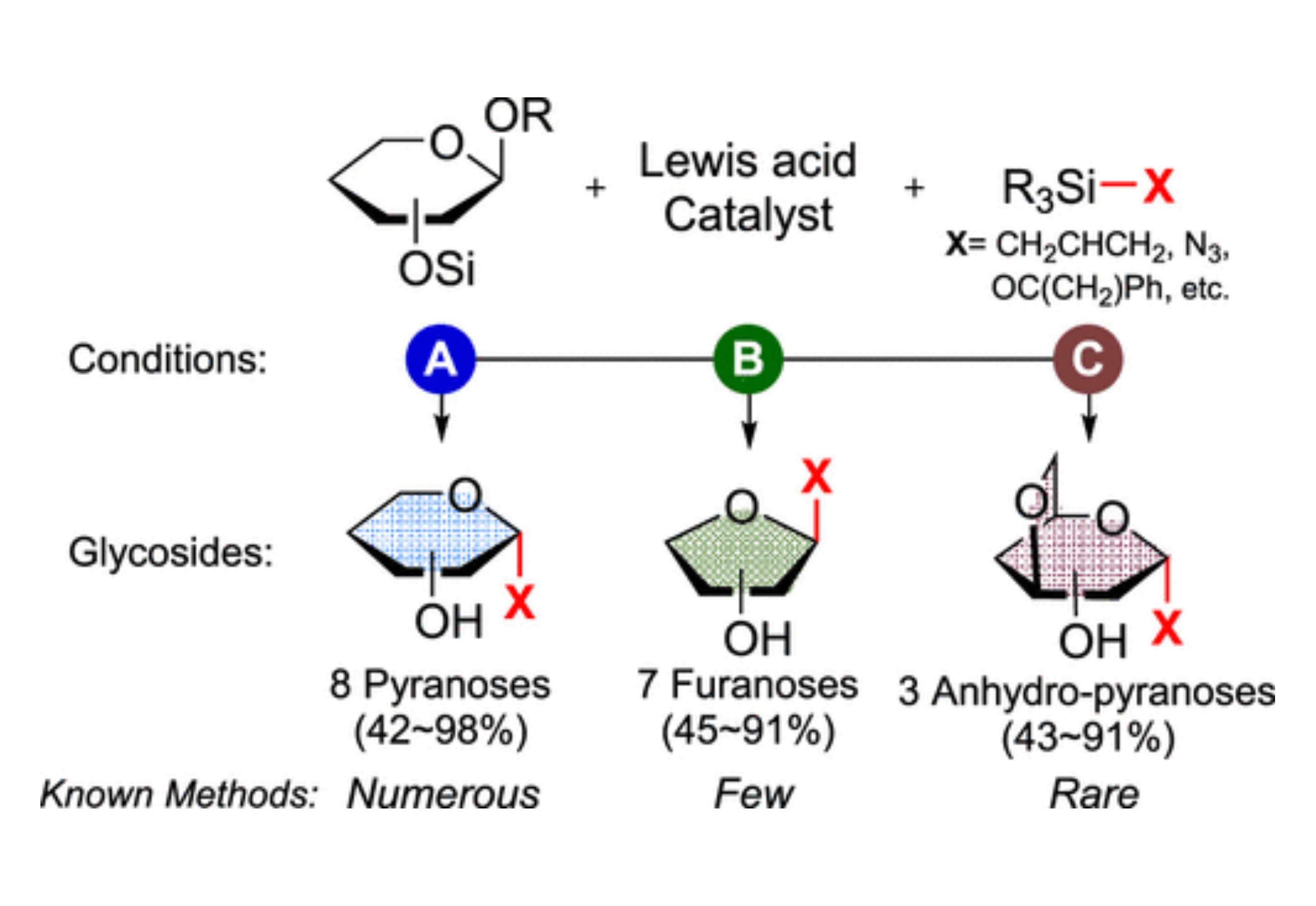
A variety of C-glycosides can be obtained from the fluoroarylborane (B(C6F5)3) or silylium (R3Si+) catalyzed functionalization of 1-MeO- and per-TMS-sugars with TMS-X reagents. A one-step functionalization with a change as simple as the addition order and/or Lewis acid and TMS-X enables one to afford chiral synthons that are common (C-pyranosides), have few viable synthetic methods (C-furanosides), or are virtually unknown (anhydro-C-pyranosides), which mechanistically arise from whether a direct substitution, isomerization/substitution, or substitution/isomerization occurs, respectively.
Citation
Switching between X-Pyrano-, X-Furano-, and Anhydro-X-pyranoside Synthesis (X = C, N) under Lewis acid Catalyzed Conditions
Youngran Seo, Jared M. Lowe, Neyen Romano, and Michel R. Gagné
Organic Letters 2021 23 (15), 5636-5640
DOI: 10.1021/acs.orglett.1c01713