Stepwise Iodide-Free Methanol Carbonylation via Methyl Acetate Activation by Pincer Iridium Complexes
Abstract
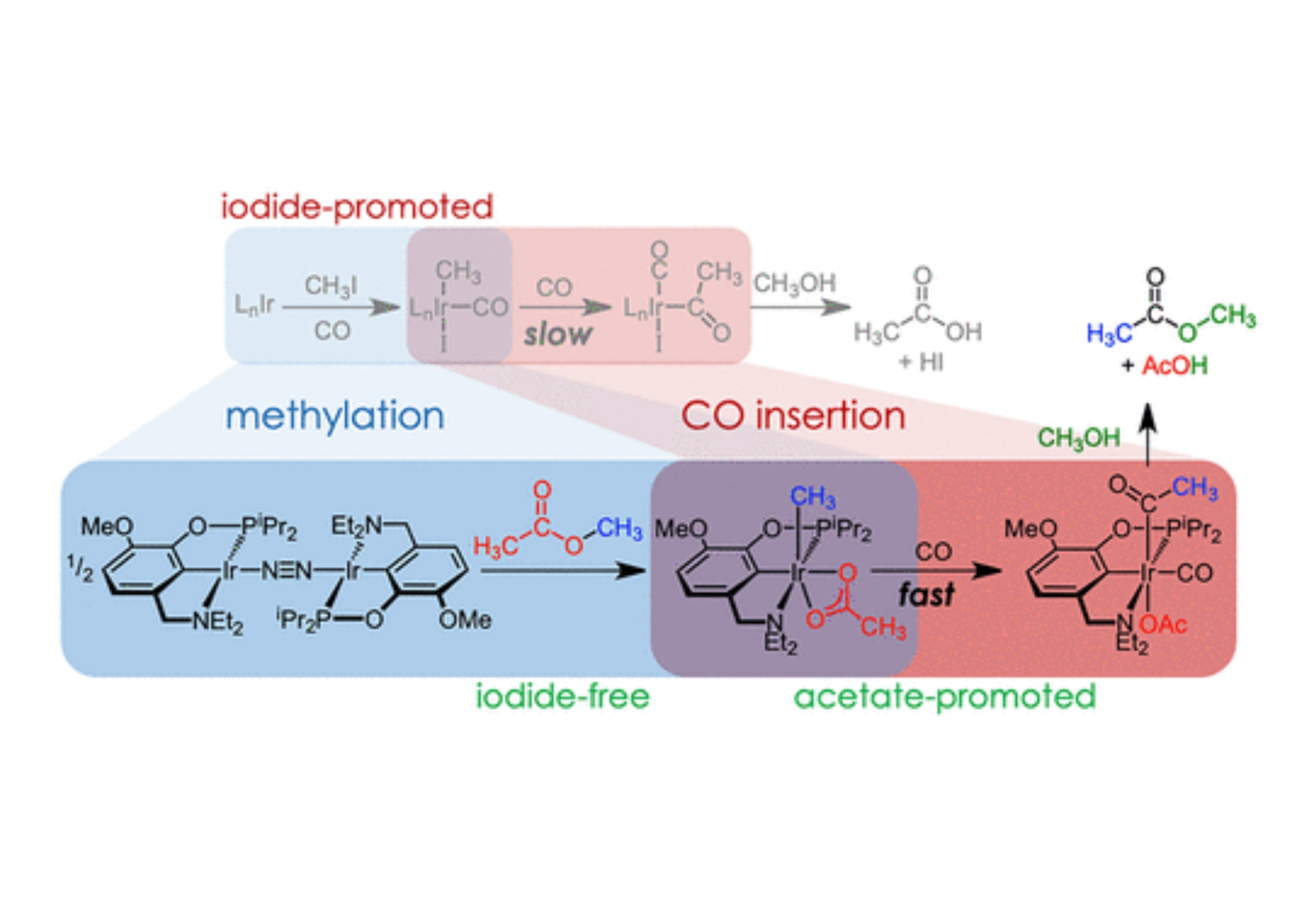
Iodide is an essential promoter in the industrial production of acetic acid via methanol carbonylation, but it also contributes to reactor corrosion and catalyst deactivation. Here we report that iridium pincer complexes mediate the individual steps of methanol carbonylation to methyl acetate in the absence of methyl iodide or iodide salts. Iodide-free methylation is achieved under mild conditions by an aminophenylphosphinite pincer iridium(I) dinitrogen complex through net C–O oxidative addition of methyl acetate to produce an isolable methyliridium(III) acetate complex. Experimental and computational studies provide evidence for methylation via initial C–H bond activation followed by acetate migration, facilitated by amine hemilability. Subsequent CO insertion and reductive elimination in methanol solution produced methyl acetate and acetic acid. The net reaction is methanol carbonylation to acetic acid using methyl acetate as a promoter alongside conversion of an iridium dinitrogen complex to an iridium carbonyl complex. Kinetic studies of migratory insertion and reductive elimination reveal essential roles of the solvent methanol and distinct features of acetate and iodide anions that are relevant to the design of future catalysts for iodide-free carbonylation.
Citation
Stepwise Iodide-Free Methanol Carbonylation via Methyl Acetate Activation by Pincer Iridium Complexes
Changho Yoo and Alexander J. M. Miller
Journal of the American Chemical Society 2021 143 (32), 12633-12643
DOI: 10.1021/jacs.1c05185