Role of Axial Ligation in Gating the Reactivity of Dimethylplatinum(III) Diimine Radical Cations
Abstract
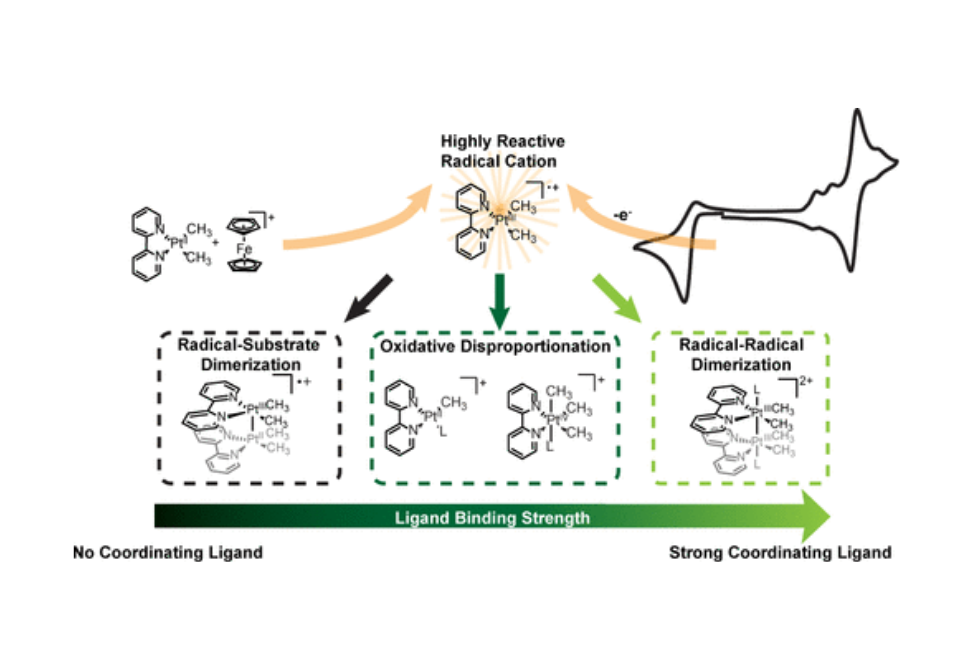
Electrochemical methods are coupled with chemical oxidant-based analytical strategies to evaluate the oxidative reactivity of the platinum(II) diimine complex (bpy)PtII(CH3)2 (bpy = 2,2′-bipyridine) in coordinating and noncoordinating media. Through this mechanistic analysis, we show that the one-electron oxidation of (bpy)PtII(CH3)2 generates a highly reactive, 15-electron PtIII radical cation and identify three reaction pathways that can follow this oxidation: radical–substrate dimerization, radical–radical dimerization, and oxidative disproportionation. Axial ligation of the initially generated [(bpy)PtIII(CH3)2]•+ species is a critical driver of this reactivity, gating the available mechanistic pathways and tuning the competition between radical–radical dimerization and oxidative disproportionation.
Citation
Role of Axial Ligation in Gating the Reactivity of Dimethylplatinum(III) Diimine Radical Cations
Brittany L. Huffman, Katherine J. Lee, Ana M. Geer, Bradley A. McKeown, Xiaofan Jia, Diane A. Dickie, T. Brent Gunnoe, and Jillian L. Dempsey
Organometallics 2021 40 (3), 333-345
DOI: 10.1021/acs.organomet.0c00663