Revealing the Molecular Identity of Defect Sites on PbS Quantum Dot Surfaces with Redox-Active Chemical Probes
Abstract
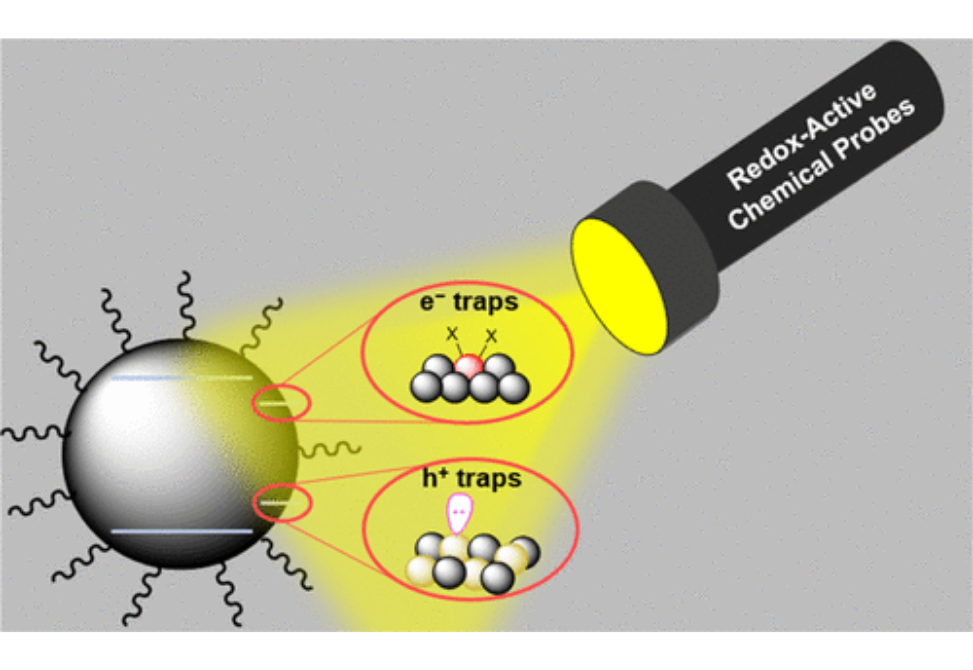
Defects arising on the surfaces of semiconductor quantum dots (QDs) limit the applications of these otherwise promising materials. Efforts to rationally passivate these sites using chemical methods, however, are limited by a lack of molecular-level understanding of surface defects. Herein, we report the application of redox-active chemical probes (E◦′ = −0.48 to −1.9 V vs Fc+/0) coupled with spectroscopic tools (nuclear magnetic resonance (NMR), X-ray photoelectron spectroscopy (XPS), and UV–vis–NIR) to gain insight into the molecular-level nature and reactivity of defects at PbS QD surfaces. First, Pb ion-based traps coordinated by oleate ligands are studied by reaction with outer-sphere reductants, wherein reduction of a subpopulation of Pb2+ ions promotes ligand displacement. We observe a correlation between this reactivity and QD size, wherein minimal ligand displacement occurs in small QDs (2.6 nm) but up to ca. 15% of ligands are displaced with larger QDs (>4 nm). The strength of the reductant also has a significant impact; with QD size held constant, more potent reductants induce a higher extent of ligand displacement than mild reductants. Finally, chalcogenide-based defects (disulfides) are interrogated with selective trialkylphosphine reagents. Comparison of QD reactivity with phosphine probes reveals that large PbS QDs possess a greater proportion of native disulfide defects than small QDs. Collectively, this work yields insight into the identities, likely structural environments and reduction potentials of targeted defect sites, thus providing a detailed picture—and roadmap for passivation—of common QD surface defects.
Citation
Revealing the Molecular Identity of Defect Sites on PbS Quantum Dot Surfaces with Redox-Active Chemical Probes
Carolyn L. Hartley and Jillian L. Dempsey
Chemistry of Materials 2021 33 (7), 2655-2665
DOI: 10.1021/acs.chemmater.1c00520