Oxidative Addition of a Phosphinite P-O Bond at Nickel
Abstract
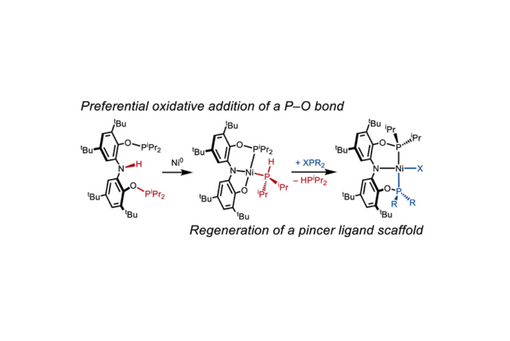
Oxidative addition is an essential elementary reaction in organometallic chemistry and catalysis. While a diverse array of oxidative addition reactions has been reported to date, examples of P–O bond activation are surprisingly rare. Herein, we report the ligand-templated oxidative addition of a phosphinite P–O bond in the diphosphinito aniline compound HN(2-OPiPr2-3,5-tBu-C6H2)2 [H(P2ONO)] at Ni0 to form (PONO)Ni(HPiPr2) after proton rearrangement. Notably, the P–O cleavage occurs selectively over an amine N–H bond activation. Additionally, the ligand cannibalization is reversible, as addition of XPR2 (X = Cl, Br; R = iPr, Cy) to (PONO)Ni(HPiPr2) readily produces either symmetric or unsymmetric (P2ONO)NiX species and free HPiPr2. Finally, the mechanisms of both the initial P–O bond cleavage and its subsequent reconstruction are investigated to provide further insight into how to target P–O bond activation.
Citation
Oxidative Addition of a Phosphinite P–O Bond at Nickel
Quinton J. Bruch, Noah D. McMillion, Chun-Hsing Chen, and Alexander J. M. Miller
Inorganic Chemistry 2023 62 (5), 2389-2393
DOI: 10.1021/acs.inorgchem.2c04188