Organosulfate Formation in Proxies for Aged Sea Spray Aerosol: Reactive Uptake of Isoprene Epoxydiols to Acidic Sodium Sulfate
Abstract
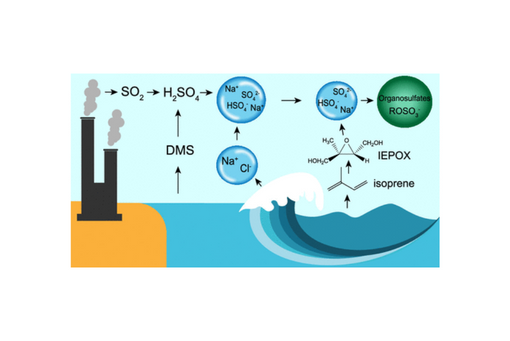
Oxidation of isoprene, the biogenic volatile organic compound (BVOC) with the highest emissions globally, is a large source of secondary organic aerosol (SOA) in the atmosphere. Particulate organosulfates formed from acid-driven reactions of the oxidation products isoprene epoxydiol (IEPOX) isomers are important contributors to SOA mass. Most studies have focused on organosulfate formation on ammonium sulfate particles, often at low pH. However, recent work has shown that sea spray aerosol (SSA) in the accumulation mode (∼100 nm) is quite acidic (pH ∼2) and undergoes further heterogeneous reactions with H2SO4 to form Na2SO4. Herein, we demonstrate that substantial SOA, including organosulfates, are formed on acidic sodium sulfate particles (pH = 1.4 ± 0.1) via controlled laboratory experiments. Comparable organosulfate formation was observed for acidic sodium and ammonium sulfate particles even though acidic particles with sodium versus ammonium as the primary cation formed less SOA volume. Both exhibited core-shell morphology after the reactive uptake of IEPOX; however, organosulfates were identified with Raman microspectroscopy in the core and shell of ammonium sulfate SOA particles, but only in the core for sodium sulfate SOA. Key organosulfates were also identified in ambient samples from the Galápagos Island. Our results suggest that isoprene-derived SOA formed on aged SSA is potentially an important, but underappreciated, source of SOA and organosulfates in marine and coastal regions that could modify SOA budgets.
Citation
Organosulfate Formation in Proxies for Aged Sea Spray Aerosol: Reactive Uptake of Isoprene Epoxydiols to Acidic Sodium Sulfate
Madeline E. Cooke, N. Cazimir Armstrong, Ziying Lei, Yuzhi Chen, Cara M. Waters, Yue Zhang, Nicolas A. Buchenau, Monica Q. Dibley, Isabel R. Ledsky, Tessa Szalkowski, Jamy Y. Lee, Karsten Baumann, Zhenfa Zhang, William Vizuete, Avram Gold, Jason D. Surratt, and Andrew P. Ault
ACS Earth and Space Chemistry 2022 6 (12), 2790-2800
DOI: 10.1021/acsearthspacechem.2c00156