On the Determination of Halogen Atom Reduction Potentials with Photoredox Catalysts
Abstract
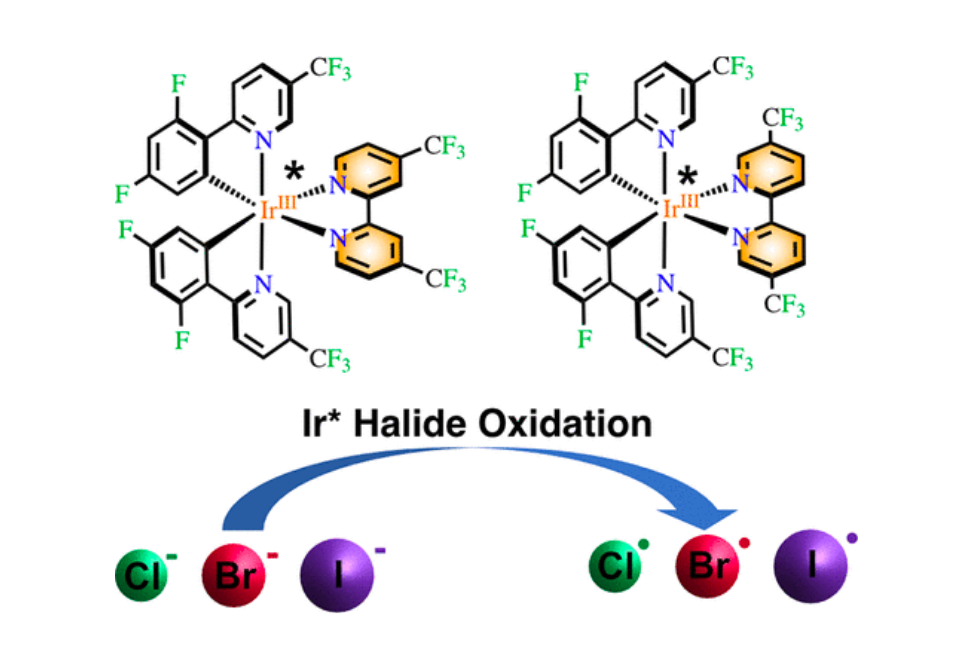
The standard one-electron reduction potentials of halogen atoms, E°′(X•/–), and many other radical or unstable species, are not accessible through standard electrochemical methods. Here, we report the use of two Ir(III) photoredox catalysts to initiate chloride, bromide, and iodide oxidation in organic solvents. The kinetic rate constants were critically analyzed through a derived diffusional model with Marcus theory to estimate E°′(X•/–) in propylene carbonate, acetonitrile, butyronitrile, and dichloromethane. The approximations commonly used to determine diffusional rate constants in water gave rise to serious disagreements with the experiment, particularly in high-ionic-strength dichloromethane solutions, indicating the need to utilize the exact Debye expression. The Fuoss equation was adequate for determining photocatalyst–halide association constants with photocatalysts that possessed +2, +1, and 0 ionic charges. Similarly, the work term contribution in the classical Rehm–Weller expression, necessary for E°′(X•/–) determination, accounted remarkably well for the stabilization of the charged reactants as the solution ionic strength was increased. While a sensitivity analysis indicated that the extracted reduction potentials were all within experimental error the same, use of fixed parameters established for aqueous solution provided the periodic trend expected, E°′(I•/–)
Citation
On the Determination of Halogen Atom Reduction Potentials with Photoredox Catalysts
Alexander M. Deetz, Ludovic Troian-Gautier, Sara A. M. Wehlin, Eric J. Piechota, and Gerald J. Meyer
The Journal of Physical Chemistry A 2021 125 (42), 9355-9367
DOI: 10.1021/acs.jpca.1c06772