Macromolecular Crowding by Polyethylene Glycol Reduces Protein Breathing
Abstract
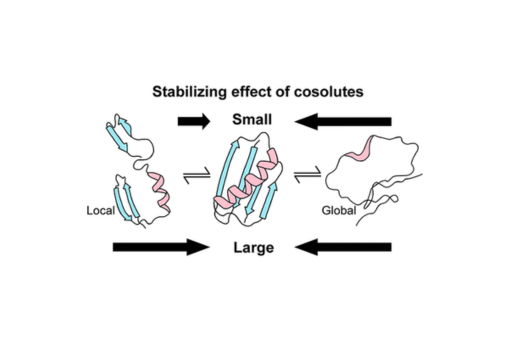
Most efforts to understand macromolecular crowding focus on global (i.e., complete) unfolding, but smaller excursions, often called breathing, promote aggregation, which is associated with several diseases and the bane of pharmaceutical and commercial protein production. We used NMR to assess the effects of ethylene glycol (EG) and polyethylene glycols (PEGs) on the structure and stability of the B1 domain of protein G (GB1). Our data show that EG and PEGs stabilize GB1 differently. EG interacts with GB1 more strongly than PEGs, but neither affects the structure of the folded state. EG and 12000 g/mol PEG stabilize GB1 more than PEGs of intermediate size, but EG and smaller PEGs stabilize GB1 enthalpically while the largest PEG acts entropically. Our key finding is that PEGs turn local unfolding into global unfolding, and meta-analysis of published data supports this conclusion. These efforts provide knowledge that can be applied to improve biological drugs and commercial enzymes.
Citation
Macromolecular Crowding by Polyethylene Glycol Reduces Protein Breathing
I-Te Chu, Brent O. Hutcheson, Hudson R. Malsch, and Gary J. Pielak
The Journal of Physical Chemistry Letters 2023 14 (10), 2599-2605
DOI: 10.1021/acs.jpclett.3c00271