Iodenium or Phosphonium: The Ambi-Valent Character of Iodophosphonium Complexes
Abstract
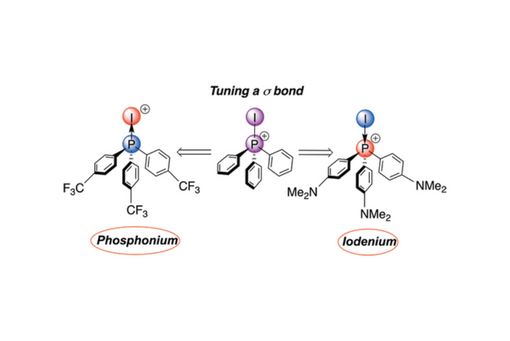
The ambi-valent character of the P–I bond in iodophosphonium complexes ensures that it can be electrophilic at either P or I. Herein, we use an ensemble of computational tools and methodologies to probe the nature of this ambi-valent bond. Geometric and atomic electron population analyses yielded strong trends between the electron donating ability of the phosphine and the strength and polarity of the P–I bond. Quasi-atomic orbital analysis demonstrated the near homo-polarity of the P–I bond, and energy decomposition analysis calculations demonstrated the ability to tune the polarization of the bond with only mild changes in secondary structural features. Finally, the ambi-valent nature of the P–I bond was demonstrated to follow hard–soft considerations in reactions with nucleophiles, with harder nucleophiles preferentially forming products of addition to P and softer nucleophiles to I.
Citation
Basemann, K., Riley, K. M., Becker, J. J., & Gagné, M. R. (2022). Iodenium or phosphonium: The ambi-valent character of iodophosphonium complexes. Inorganic Chemistry, 61(44), 17550–17556. https://doi.org/10.1021/acs.inorgchem.2c02543