Efficient Enantio-, Diastereo, E/Z-, and Site-Selective Nickel-Catalyzed Fragment Couplings of Aldehydes, Dienes, and Organoborons
Abstract
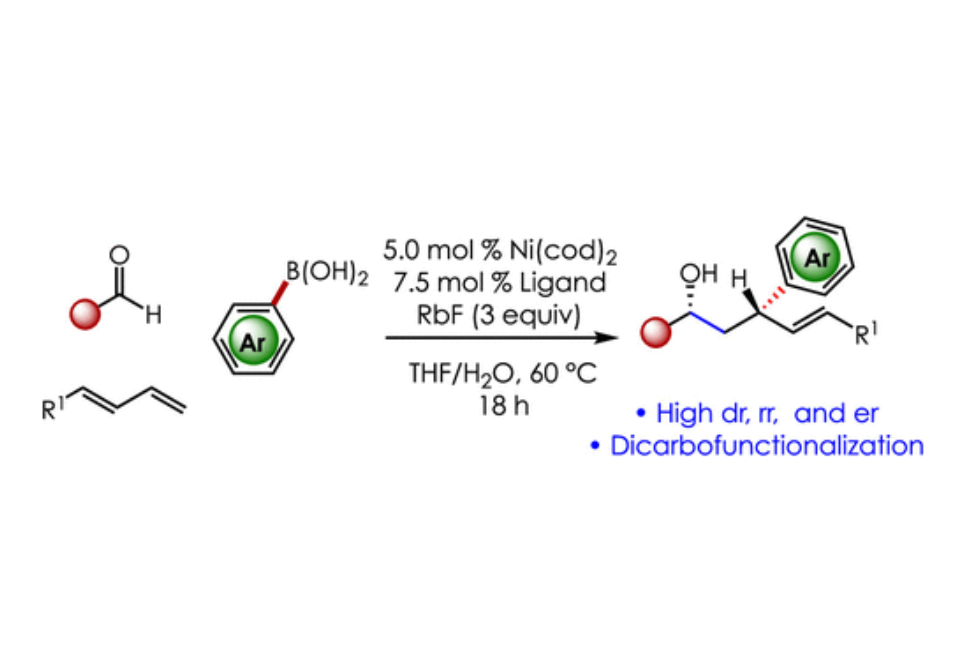
The enantioselective synthesis of bis-homoallylic alcohols through nickel-catalyzed three-component fragment couplings of simple aldehydes, dienes, and aryl organoborons is disclosed. The reactions proceed through diene dicarbofunctionalization that concurrently forms two C–C bonds and two stereogenic centers. The transformations are promoted by a 5.0 mol % loading of a readily accessible chiral phosphine–nickel complex and afford products with high stereoselectivity.
Citation
Efficient Enantio-, Diastereo, E/Z-, and Site-Selective Nickel-Catalyzed Fragment Couplings of Aldehydes, Dienes, and Organoborons
Justin S. Marcum and Simon J. Meek
Journal of the American Chemical Society Article ASAP
DOI: 10.1021/jacs.2c08742