Effects of Ligand Shell Composition on Surface Reduction in PbS Quantum Dots
Abstract
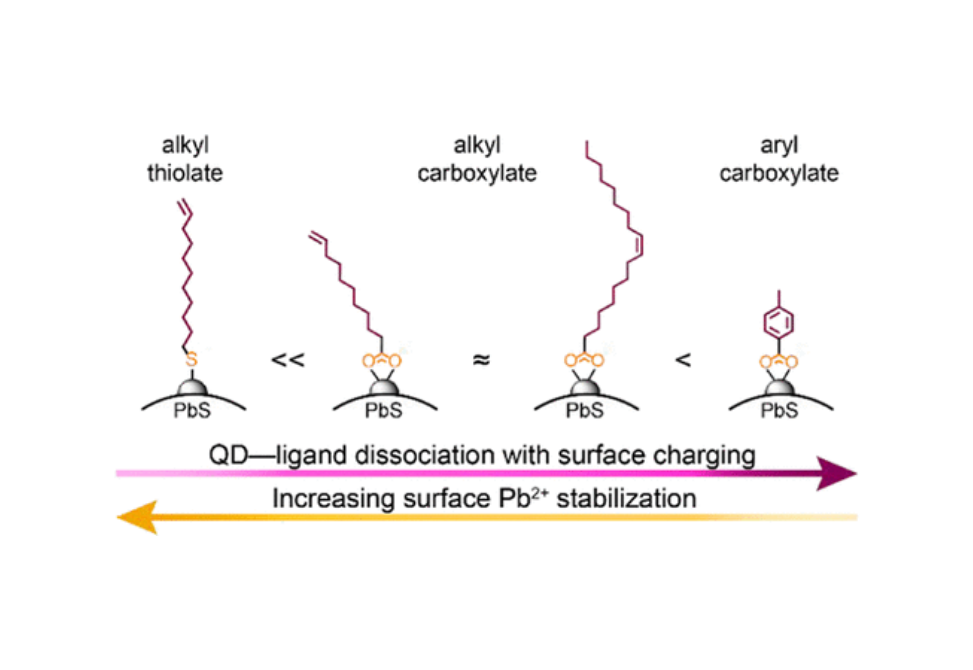
Quantum dots (QDs) are semiconductor nanocrystals with optical properties that can be tuned through postsynthetic ligand exchanges. Importantly, the stability of QD surfaces in optoelectronic devices is influenced by the ligand shell composition and the structure of the exchange ligand. QDs incorporated into such devices are frequently exposed to excess electronic charges that can localize at the surface via doping, charge hopping, etc. However, changes in the reactivity and stability of QDs upon surface reduction as a function of ligand shell composition are not well understood. In this work, we evaluated the impacts of both surface-binding head group and ligand backbone on the properties and reactivity of PbS QDs through a partial exchange of native oleate ligands with undec-10-enoic acid, p-toluate, and undec-10-ene-1-thiol to access mixed-shell QDs. We compared the reactivity and stability of these mixed-shell QDs in response to surface reduction via the addition of a molecular reductant, cobaltocene (CoCp2). Upon reaction with CoCp2, X-type ligand displacement from the QD surface was observed in each of the mixed-shell systems and monitored via 1H NMR spectroscopy. Comparative studies reveal that indiscriminate and moderate ligand displacement occurs from QDs capped with long-chain carboxylate ligands (ca. 10% ligands displaced), while more dramatic (ca. 20–30%) and preferential displacement of aryl ligands occurs with a mixed shell of alkyl and aryl carboxylates. In contrast, QDs capped with a mix of thiolate, thiol, and carboxylate ligands only exhibit displacement of carboxylate ligands. Overall, this work demonstrates that the extent of surface reduction induced by the addition of a molecular reductant is highly sensitive to the composition of the QD ligand shell.
Citation
Effects of Ligand Shell Composition on Surface Reduction in PbS Quantum Dots
Carolyn L. Hartley, Melody L. Kessler, Christian Y. Dones Lassalle, Andrew M. Camp, and Jillian L. Dempsey
Chemistry of Materials 2021 33 (22), 8612-8622
DOI: 10.1021/acs.chemmater.1c01810