Doubly stereoconvergent crystallization enabled by asymmetric catalysis
Abstract
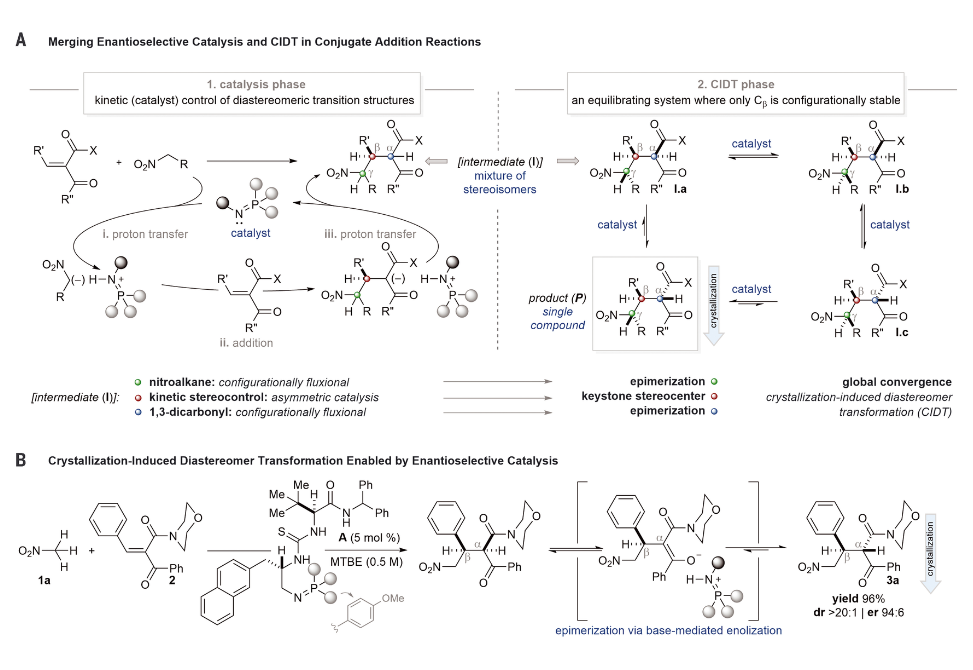
Synthetic methods that enable simultaneous control over multiple stereogenic centers are desirable for the efficient preparation of pharmaceutical compounds. Herein, we report the discovery and development of a catalyst-mediated asymmetric Michael addition/crystallization–induced diastereomer transformation of broad scope. The sequence controls three stereogenic centers, two of which are stereochemically labile. The configurational instability of 1,3-dicarbonyls and nitroalkanes, typically considered a liability in stereoselective synthesis, is productively leveraged by merging enantioselective Brønsted base organocatalysis and thermodynamic stereocontrol using a single convergent crystallization. The synthesis of useful γ-nitro β-keto amides containing three contiguous stereogenic centers is thus achieved from Michael acceptors containing two prochiral centers.
Citation
de Jesús Cruz, P.; Cassels, W. R.; Chen, C.; Johnson, J. S. Doubly Stereoconvergent Crystallization Enabled by Asymmetric Catalysis. Science 2022, 376, 1224– 1230, DOI: 10.1126/science.abo5048