Divergent Functionalization of Alkynes Enabled by Organic Photoredox Catalysis
Abstract
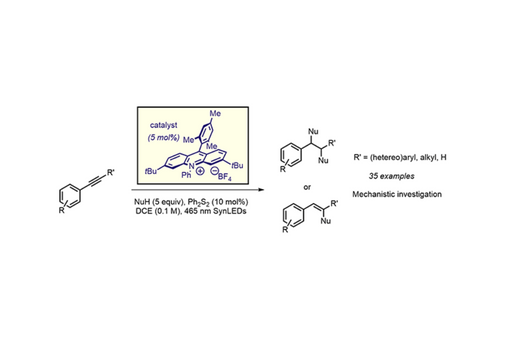
Direct functionalization of alkynes under oxidative conditions is challenging, as alkynes are usually recalcitrant towards typical oxidants. Herein, we communicate a strategy for the divergent functionalization of alkynes with photoexcited acridinium organic dyes, presumably via the formation of vinyl cation radicals as key intermediates. Based on the nature of the nucleophiles, different types of difunctionalized products were obtained in moderate to good yields. Addition of lithium Lewis acids resulted in a surprising reversal of diastereocontrol.
Citation
Nicewicz, D. A., Zhu, Z., & Qian, S. (2023). Divergent functionalization of alkynes enabled by organic photoredox catalysis. Synlett. https://doi.org/10.1055/a-2009-8279