Catalytic Dehydrogenation of Alkanes by PCP-Pincer Iridium Complexes Using Proton and Electron Acceptors
Abstract
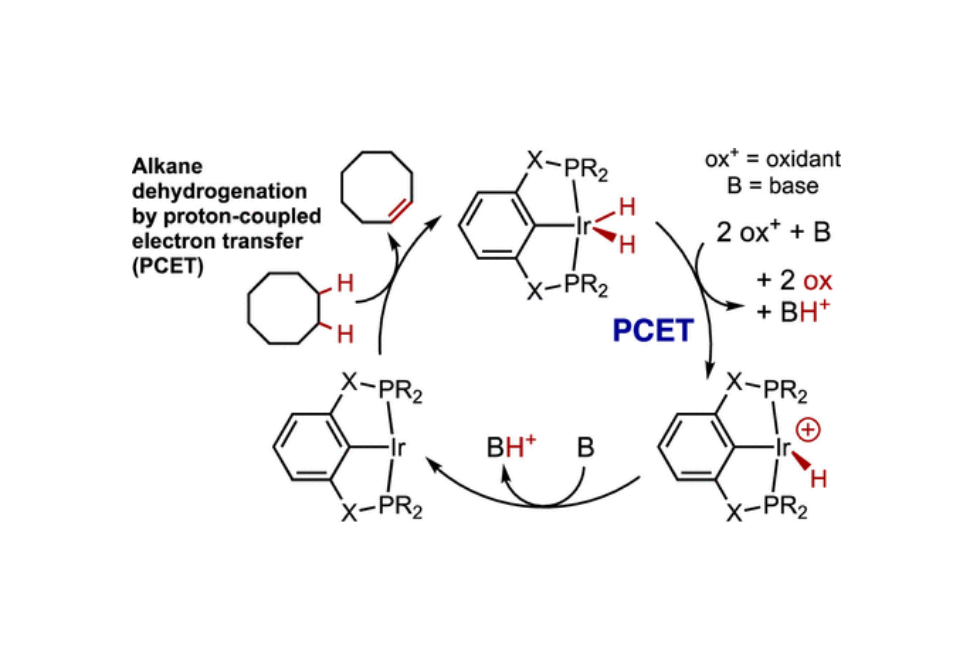
Dehydrogenation to give olefins offers the most broadly applicable route to the chemical transformation of alkanes. Transition-metal-based catalysts can selectively dehydrogenate alkanes using either olefinic sacrificial acceptors or a purge mechanism to remove H2; both of these approaches have significant practical limitations. Here, we report the use of pincer-ligated iridium complexes to achieve alkane dehydrogenation by proton-coupled electron transfer, using pairs of oxidants and bases as proton and electron acceptors. Up to 97% yield was achieved with respect to oxidant and base, and up to 15 catalytic turnovers with respect to iridium, using t-butoxide as base coupled with various oxidants, including oxidants with very low reduction potentials. Mechanistic studies indicate that (pincer)IrH2 complexes react with oxidants and base to give the corresponding cationic (pincer)IrH+ complex, which is subsequently deprotonated by a second equivalent of base; this affords (pincer)Ir which is known to dehydrogenate alkanes and thereby regenerates (pincer)IrH2.
Citation
Catalytic Dehydrogenation of Alkanes by PCP–Pincer Iridium Complexes Using Proton and Electron Acceptors
Arun Dixith Reddy Shada, Alexander J. M. Miller, Thomas J. Emge, and Alan S. Goldman
ACS Catalysis 2021 11 (5), 3009-3016
DOI: 10.1021/acscatal.0c05160