Biosynthetic Origin of Formylaminooxyvinylglycine and Characterization of the Formyltransferase GvgI
Abstract
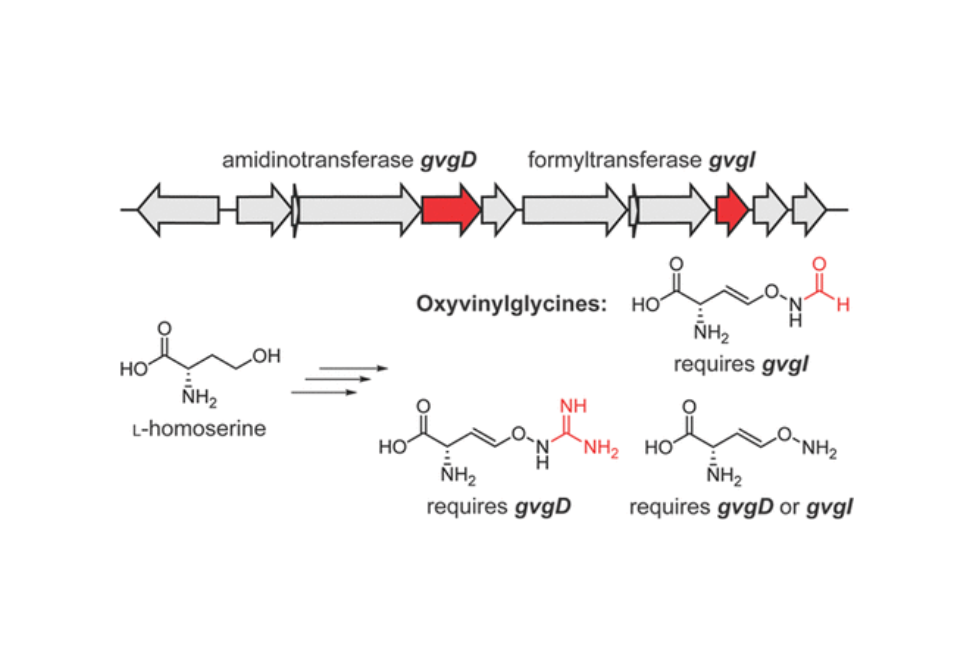
4-Formylaminooxyvinylglycine (FVG) is an herbicidal and antibacterial nonproteinogenic amino acid produced by several strains of the Pseudomonas fluorescens species complex. It contains a unique vinyl alkoxyamine moiety with an O–N bond, and its biosynthetic origin remains unknown. Here, we show that the gvg cluster from P. fluorescens WH6 is responsible for the biosynthesis of FVG and two additional O–N bond-containing oxyvinylglycines, guanidinooxyvinylglycine and aminooxyvinylglycine. Feeding studies in the producing bacteria indicate that these compounds originate from homoserine. We identify a formyltransferase gvgI that is required for the production of FVG and characterize the activity of this enzyme in vitro toward amino acids with a side chain amine. Sequence similarity network analysis reveals that GvgI and homologues make up a distinct group from the main classes of formyltransferases.
Citation
Biosynthetic Origin of Formylaminooxyvinylglycine and Characterization of the Formyltransferase GvgI
Adam R. Lescallette, Zachary D. Dunn, Viola A. Manning, Kristin M. Trippe, and Bo Li
Biochemistry 2022 61 (19), 2159-2164
DOI: 10.1021/acs.biochem.2c00374