A Catalytic Method for the Enantioselective Synthesis of alpha-Quaternary Ketones, alpha-Ketoesters and Aldehydes
Abstract
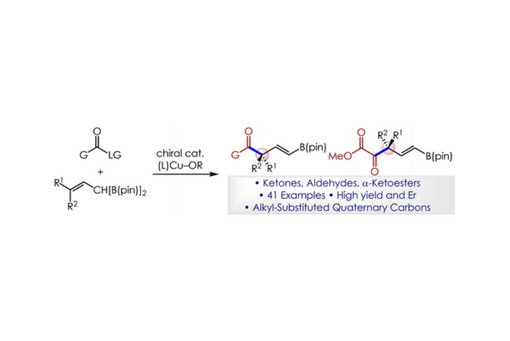
A practical method for the efficient and enantioselective preparation of versatile ketones and aldehydes that contain an α-quaternary stereocenter is described. Reactions utilize simple carboxylic acid or ester starting materials, a monodentate chiral phosphine, and afford a variety of aryl, alkenyl, alkynyl, and alkyl-substituted ketone and aldehyde products in 25–94 % yield and 90 : 10 to >99 : 1 enantiomeric ratio. Reactions proceed by acyl substitution with in situ formed chiral allylic nucleophiles, and display selectivity and conversion dependence on a protic additive. The utility of the approach is demonstrated through several product transformations.
Citation
Wheatley, E., Zanghi, J. M., Mason, M. M., & Meek, S. J. (2023). A catalytic method for the enantioselective synthesis of α‐quaternary ketones, α‐ketoesters and aldehydes. Angewandte Chemie. https://doi.org/10.1002/ange.202215855