11C-, 12C-, and 13C-cyanation of electron-rich arenes via organic photoredox catalysis
Abstract
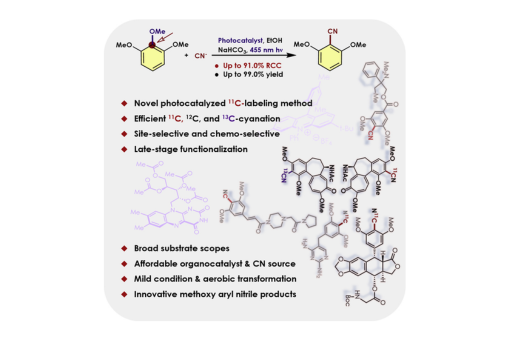
As a non-invasive imaging technology, positron emission tomography (PET) plays a crucial role in personalized medicine, including early diagnosis, patient screening, and treatment monitoring. The advancement of PET research depends on the discovery of new PET agents, which requires the development of simple and efficient radiolabeling methods in many cases. As bioisosteres for halogen and carbonyl moieties, nitriles are important functional groups in pharmaceutical and agrochemical compounds. Here, we disclose a mild organophotoredox-catalyzed method for efficient cyanation of a broad spectrum of electron-rich arenes, including abundant and readily available veratroles and pyrogallol trimethyl ethers. Notably, the transformations not only are compatible with various affordable 12C- and 13C-cyanide (CN) sources but also could be applied to carbon-11 synthons to incorporate [11C]nitriles into arenes. The aryl [11C]nitriles can be further derivatized to [11C]carboxylic acids, [11C]amides, and [11C]alkyl amines. The newly developed reaction can serve as a powerful tool for generating new PET agents.
Citation
Wu, X., Chen, W., Holmberg-Douglas, N., Bida, G. T., Tu, X., Ma, X., Wu, Z., Nicewicz, D. A., & Li, Z. (2023). 11C-, 12C-, and 13C-cyanation of electron-rich arenes via organic photoredox catalysis. Chem, 9(2), 343–362. https://doi.org/10.1016/j.chempr.2022.12.007