Study: Cadmium Molecules Improve Light-Emitting Properties of Tiny Particles
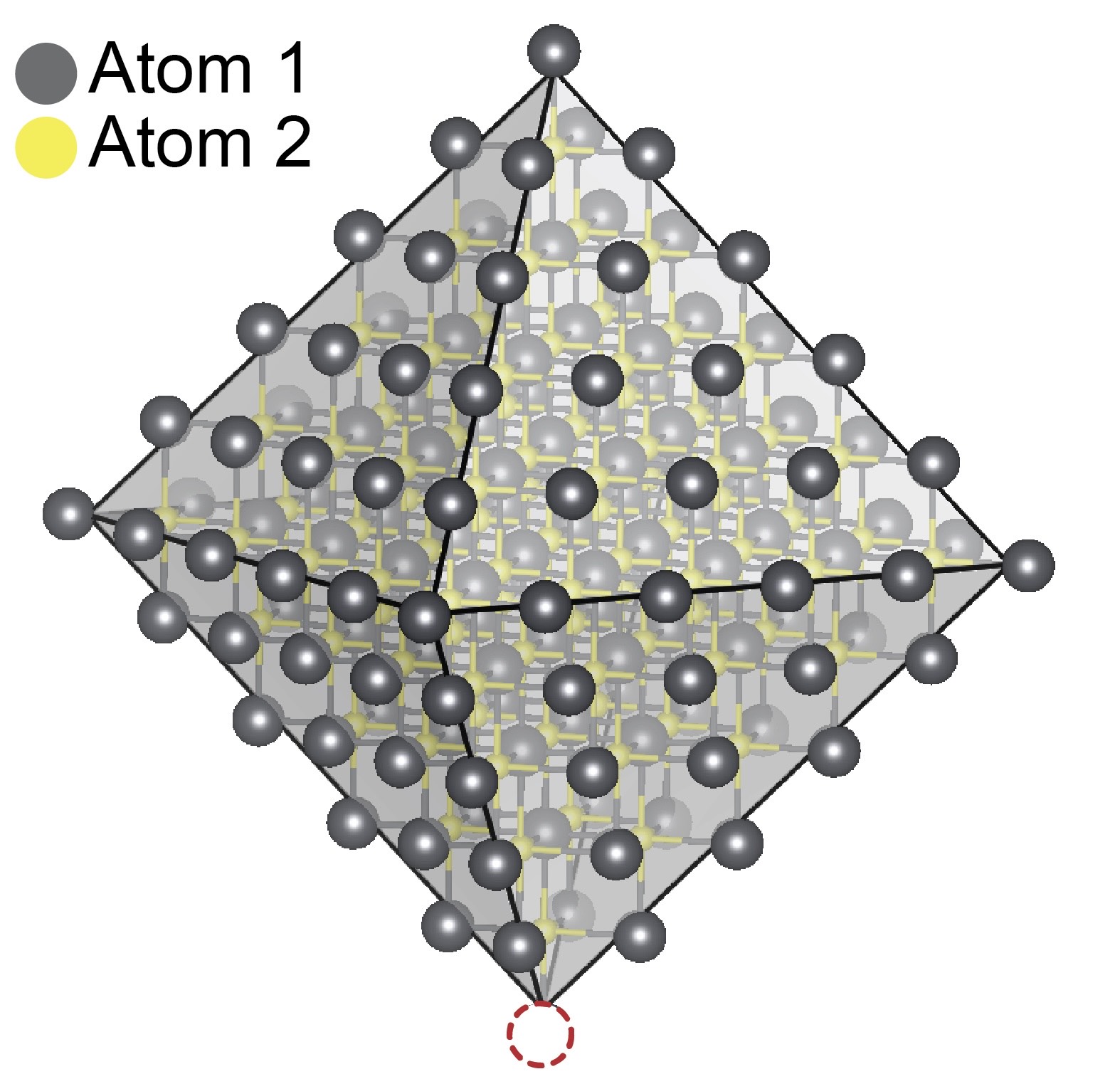
The dotted circle shows a missing a molecule, called a ligand, from a nanometer-size quantum dot. This defect can compromise the quantum dot's ability to emit light.
March 12, 2024 | By Dave DeFusco

Special molecules called Z-type ligands can fix defects on quantum dots, the nanometer-size particles used to tune the colors in LED lights and improve the brilliance of TV screens, but they don’t always optimize their electronic or optical properties, according to a paper by UNC-Chapel Hill chemistry researchers in the Journal of the American Chemical Society.
“Our research is focused on understanding the origin of defects on the surface of semiconductor quantum dots and to fix these defects,” said Jennica Kelm, a Ph.D. student and first author of the paper, “Metal-Dictated Reactivity of Z-Type Ligands to Passivate Surface Defects on CdSe Nanocrystals.“ “These defects are generally deleterious to optical or electronic properties that we want to optimize.”
Quantum dots are just a few nanometers in diameter and have no dimensionality, meaning they have no length, width or height. A nanometer is a billionth of a meter, or one 25-millionth of an inch—bigger than an atom or simple molecule. For perspective, the difference in size between a quantum dot and a soccer ball is about the same as the difference between a soccer ball and Earth.

Ligands stick to the quantum dot by sharing some of their electrons, forming a bond. Z-type ligands, said Kelm, are like little metal-based companions that cozy up to the non-metal ions on the quantum dot and influence how it behaves, or what kinds of reactions it can undergo.
When the surface of the quantum dot is missing Z-type ligands, a defect is created that reduces the dot’s efficiency and, in some cases, prevents it from emitting light. The researchers then experimented with replacing the missing Z-type ligand with combinations of other non-native Z-type ligands based on metals, such as cadmium, zinc and lead, to test their ability to fix the defect.
“Just because we can put a Z-type ligand back on the surface of the quantum dot doesn’t mean it fixes all of our problems,” said Kelm. “Even if we have a perfectly symmetrical quantum dot that is nominally defect-free, it still might not have the electronic properties that we desire. What we did provides design principles for how one might go about fixing quantum dots that have defects so that their optical and electronic properties are optimized for a TV or solar cell.”
Jillian Dempsey, co-author of the paper and Bowman and Gordon Gray Distinguished Term Professor, said their work takes an experimental approach toward understanding the differences between chemical passivation, which makes the ligands stick to the surface of the quantum dot, and electronic passivation, which makes the quantum dot light up again.
She added that the study is the first to reveal how Z-type ligands behave when interacting with semiconductor quantum dots, thanks to combining techniques called nuclear magnetic resonance spectroscopy with photoluminescence spectroscopy. Even though all Z-type ligands attach to the surface of cadmium selenide quantum dots, only cadmium-based ligands cause a significant increase in the light emission’s brightness. This suggests that while zinc and lead Z-type ligands can help cover up imperfections on the surface of cadmium selenide quantum dots, they don’t fully get rid of certain defects that can affect how well they emit light.
“While both chemical and electronic passivation can lead to improved performance of semiconductor quantum dots, they operate through different mechanisms and address different aspects of the material’s properties,” said Dempsey, who is also deputy director of the Center for Hybrid Approaches in Solar Energy to Liquid Fuels. “Controlling the surface chemistry is imperative to achieving defect-free surfaces of quantum dots, but the impact of chemical surface-passivation by a variety of metals on quantum dots’ electronic properties has been poorly studied and the relationship between chemical and electronic passivation is not established.”
Kelm said the ultimate goal of their research is to create a near-perfect material that could be used in solar cells, solid-state lighting and TV and computer displays.
“As researchers begin to integrate quantum dots into next-generation devices,” she said, “our work will inform the relevant surface modification procedures necessary for electronically robust and highly luminescent quantum dots.”

