November 17, 2023 | By UNC-Chapel Hill Chemistry Communication
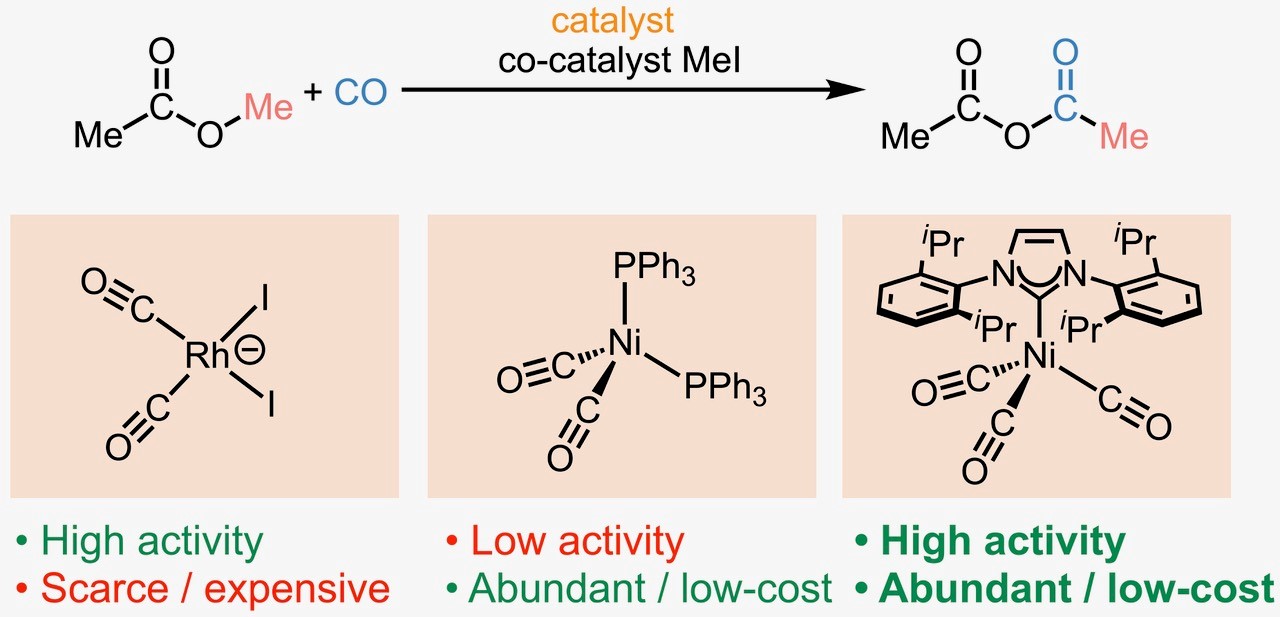
UNC-Chapel Hill chemist Alex Miller and Eastman scientist Javier Grajeda are leading a collaborative academic-industry team investigating more sustainable routes to elaborate basic building blocks of the chemical industry. Currently in the chemical industry, compounds such as methanol and methyl esters are converted to acetic acid or acetic anhydrides that are eventually incorporated into plastics, fabrics, medicines, and other products. Millions of tons of these chemical intermediates are prepared by carbonylation reactions, in which a carbon monoxide unit is added to a compound, but these reactions traditionally rely on expensive and scarce precious metal catalysts such as rhodium and iridium to accelerate the reaction.
A new reaction process offers a potential alternative. In a study published in Science, the Miller Lab and Eastman scientists report that nickel, when paired with organic imidazole derivatives, is an excellent catalyst system for carbonylation reactions. Nickel is a much more affordable, abundant, and environmentally responsible metal source than rhodium. In prior studies of nickel carbonylation catalysis, which employed phosphine ligands, the rate of product formation was low, even when large amounts of catalyst and promoters were used.
A historical quirk provided the opportunity the researchers needed to discover this breakthrough. Just as interest in nickel-catalyzed carbonylation was waning due to the poor performance, in other fields of chemistry a new class of promoting ligands called N-heterocyclic carbenes (NHCs) was emerging. Thus, inspired by this historical missed opportunity to pair nickel and NHCs, the research team re-examined the nickel-catalyzed carbonylation of esters to valuable anhydride compounds supported by imidazole derivatives.
This combination has proved to be an extraordinary catalyst system, with high activity even at low loadings of nickel, ligand, and additives. The catalyst system is tolerant to air and can be recharged with carbon monoxide without major losses in activity. And another significant benefit is that nickel costs significantly less than rhodium. For reference, a troy ounce of rhodium can cost as much as a metric ton of nickel. The rates of the new nickel system invite comparisons to current state-of-the-art precious metal catalysts currently used in industry, which opens the door for further exploration and optimization.
This research was made possible by a longstanding research partnership between UNC and Eastman. Recognizing the alignment of UNC’s expertise in catalyst development and polymer chemistry with Eastman’s innovation endeavors, a master research agreement was established in 2013. Over the past 10 years, Eastman has funded 38 sponsored research projects, totaling over $7 million and spanning across five departments at UNC.
One of the first of these projects aimed at innovating homogeneous carbonylation catalysis, which eventually led to this new discovery of the new nickel system for anhydride synthesis. Two patents have been filed on this process so far. This sets the stage to further explore nickel-catalyzed ester carbonylation.
Developing and optimizing a continuous process, guided by technoeconomic and life-cycle assessments, will unlock the full potential of this chemistry. This fruitful partnership highlights the value of addressing industrially relevant problems when viewed through multiple lenses — from fundamental mechanistic insights to industrial scalability considerations.
Founded in 1920, Eastman is a global specialty materials company that produces a broad range of products found in items people use every day. With the purpose of enhancing the quality of life in a material way, Eastman works with customers to deliver innovative products and solutions while maintaining a commitment to safety and sustainability. The company’s innovation-driven growth model takes advantage of world-class technology platforms, deep customer engagement, and differentiated application development to grow its leading positions in attractive end-markets such as transportation, building and construction, and consumables. As a globally inclusive and diverse company, Eastman employs approximately 14,500 people around the world and serves customers in more than 100 countries. The company had 2022 revenues of approximately $10.6 billion and is headquartered in Kingsport, Tennessee, USA. For more information, visit www.eastman.com.
To read the published article in Science, please click the Read More below.