First-Principles Approach for Coupled Quantum Dynamics of Electrons and Protons in Heterogeneous Systems
January 24, 2024 | By UNC-Chapel Hill Chemistry Communication
Kanai group has recently published a paper in Physical Review Letters (PRL), the issue 23/2023, which has been selected as an Editor’s Suggestion. This issue of Physical Review Letters (PRL) features the article “First-Principles Approach for Coupled Quantum Dynamics of Electrons and Protons in Heterogeneous Systems” by Professor Yosuke Kanai and his colleagues.
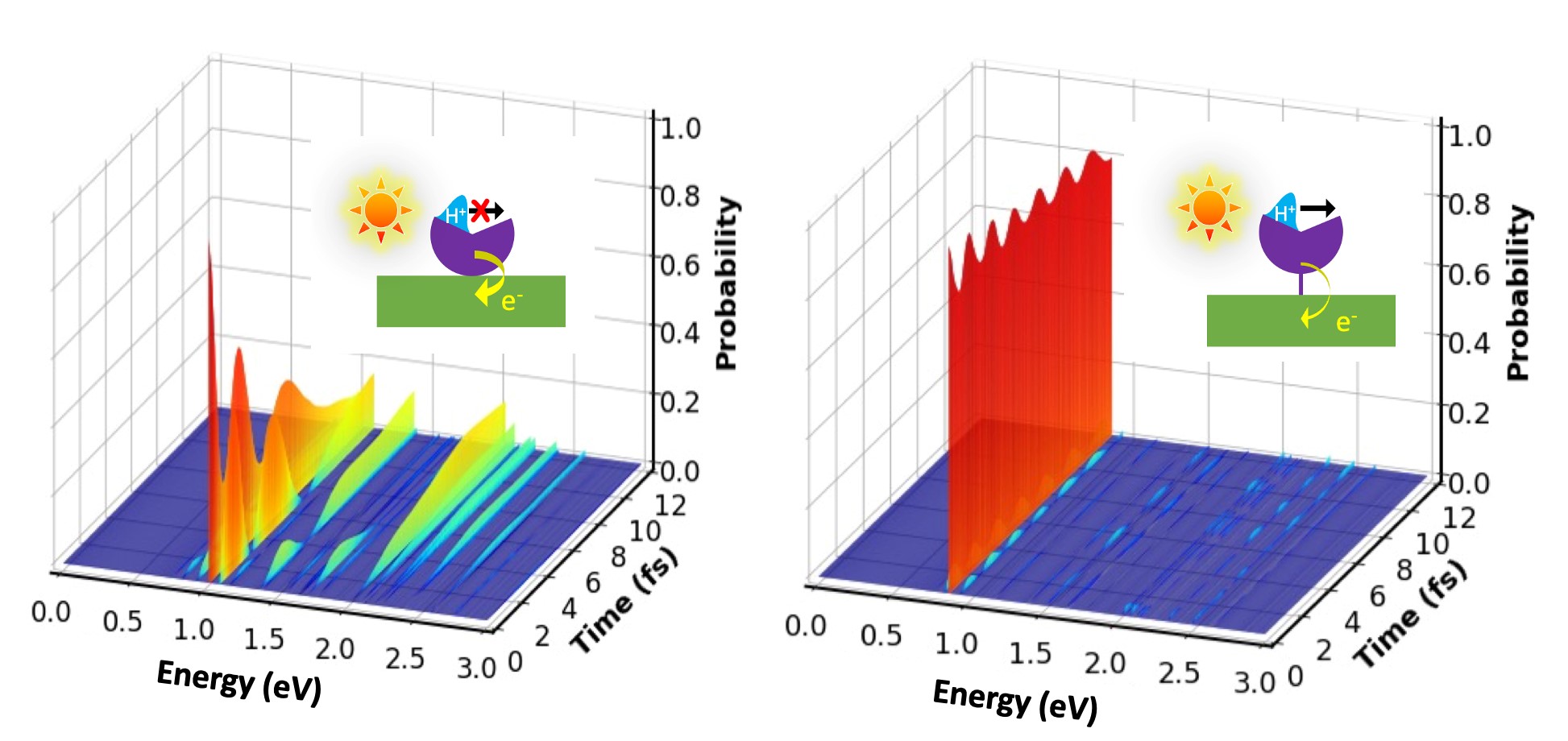
The coupled quantum dynamics of electrons and protons is ubiquitous in many dynamical processes involving light-matter interaction, such as solar energy conversion in photosynthesis. A first-principles description of such nuclear-electronic quantum dynamics requires not only the explicit time-dependent treatment of nonequilibrium electron dynamics but also that of quantum protons. Quantum mechanical correlation between electrons and protons adds further complexity to such coupled dynamics. New real-time nuclear-electronic orbital time-dependent density functional theory was developed and demonstrated for studying coupled quantum dynamics of electrons and protons in complex heterogeneous systems from first principles. The process studied is an electronically excited-state intramolecular proton transfer in water and at a semiconductor-molecule interface. These simulations illustrate how environments such as hydrogen-bonding water molecules and an extended material surface impact the coupled quantum dynamical process on the atomistic level. Depending on how the molecule is adsorbed on the surface, excited-state electron transfer from the molecule to the semiconductor surface can inhibit ultrafast proton transfer within the molecule. This letter elucidates how heterogeneous environments influence the balance between quantum mechanical proton transfer and excited electron dynamics. The work was supported by the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Basic Energy Sciences, Office of Science, under Award No. DESC0021173.
To read the published article, please click Read More below.

