Electrochemist tracks protons and electrons to improve alternative fuel production

Montgomery, the study’s lead author, weighs the catalyst in an air-free glovebox.
March 11, 2025 | By Emily Sherman
UNC-Chapel Hill Chemistry student Charlotte Montgomery investigates the smallest details, and that’s no exaggeration: the electrochemist tracks the movements of protons and electrons trillions of times smaller than a grain of sand to advance sustainable energy technology. For her contributions to the field, Montgomery was one of four students recognized with the Francis Preston Venable Chemistry Scholarship.
As a member of the Dempsey Group, Montgomery investigates how molecular catalysts precisely move protons and electrons to drive chemical reactions and produce alternative fuels. As these catalysts mediate electrochemical transformations, protons and electrons migrate from earth abundant feedstocks, like water to the catalyst and form fuels like hydrogen gas. Identifying the pathway and measuring the rate of these transfers is crucial to determine how efficiently the desired fuel is formed. If Montgomery and others can better understand the proton and electrons’ movements, they can better tune the catalysts’ structures to produce the desired fuel more efficiently.
“Compared to traditional material catalysts, molecular catalysts that mediate electrochemical reactions offer new avenues for understanding what’s really going on at an atomic level,” said Montgomery. “That’s key for understanding why catalysts break down or participate in non-productive reactions, and we can use that information to further improve catalyst performance.”
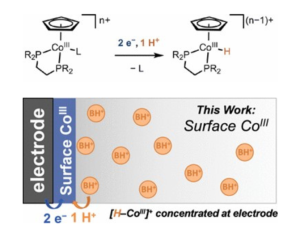
To achieve this, Montgomery and coworkers have measured the rate of the proton-coupled electron transfer of catalysts anchored to a surface. By tracking how quickly protons moved through the catalyst in different conditions, the chemists could calculate the proton transfer’s rate — ten thousand times faster than the blink of an eye. With rising global CO2 levels, this new analytical method comes at a critical time in alternative fuel development and could expedite the path to more efficient catalytic technologies.
“Charlotte has pushed forward our understanding of how proton transfers to metal centers are not simply dictated by thermodynamics, but also by the kinetic barriers associated with these elementary reaction steps. She’s mapped out how transition metal complexes take circuitous, multi-step routes to avoid individual steps with high kinetic barriers, no matter how much driving force there is. This understanding is providing a blueprint for catalyst design,” said Dr. Jillian Dempsey, Bowman and Gordon Gray Distinguished Term Professor and Deputy Director of the Center for Hybrid Approaches in Solar Energy to Liquid Fuels. “Furthermore, she’s the first person to quantify a rate constant for a proton-coupled electron transfer reaction of a surface immobilized complex using cyclic voltammetry, a critical advance for realizing integrated catalyst systems!”
Outside of the laboratory, Montgomery connected UNC students with industry leaders as a member of the Graduate Committee for Professional Development (GCPD) and an organizer of Chemistry’s annual Industry InSight. The event’s year-long planning process culminated in a day of presentations, workshops, and networking that fostered new relationships for her peers. For her work advancing these technologies and connecting the chemistry community, Montgomery was recognized with the Francis Preston Venable Chemistry Scholarship.
“Working with the supportive community at UNC, including the GCPD, has filled my cup in ways I didn’t know were possible,” said Montgomery. “These past five years have driven home the importance of a supportive community for developing creative solutions to our global challenges. Looking back, I see how understanding the smallest details, from proton transfers to smiling at people in the hallways, can cultivate the innovation and community we need to impact our world.”

