Gerald Meyer
Professor
Murray Hall 2202F919-843-8313
gjmeyer@email.unc.edu
Group Website
Curriculum Vitae
Research Interests
Synthetic chemistry, Spectroscopy, and Electrochemistry
Research Synopsis
Transition metal compounds often possess low-lying excited states that can be populated with visible light. The nature of these excited states is of fundamental interest in their own right as is there subsequent reactivity. Particular emphasis on the excited states has focused on:
-
- Quantification of light driven inner-sphere reorganization that is novel to copper phenanthroline compounds by photoluminescence and, more directly, by x-ray spectroscopies. The x-ray characterization represents an ongoing collaboration with Lin Chen and her coworkers at the Advanced Photon Source, Argonne National Laboratory.
-
- Identifying the molecular origin(s) of the activational barriers for internal conversion of charge-transfer and ligand-field excited states in Ru polypyridyl compounds in fluid solution and at semiconductor interfaces.
-
- Prevention of the rapid light induced excited state spin trapping by the 5T state of spin-crossover Fe(II) benzimidazole compounds that occurs at TiO2 interfaces.
-
- Characterization of Co(I) compounds with low-lying metal-to-ligand charge transfer excited states.
- Dissociative excited states that release CO will be exploited to measure dioxygen activation by copper on short time scales. This research represents an ongoing collaboration with Ken Karlin and his coworkers at Johns Hopkins.
An excited state reaction of particular relevance to energy conversion in dye-sensitized solar cells is electron transfer to the unfilled acceptor states of TiO2 nanocrystallites. A fundamental goal is to optimize the efficiency of this reaction while at the same time preventing the unwanted charge recombination reaction. Specific goals include:
-
- Optimization of the relative rate constants for excited state injection and charge recombination by introduction of a conjugated bridge between a Ru polypyridyl compound and the surface anchoring groups. This research is done in collaboration with Elena Galoppini and her coworkers at Rutgers-Newark.
-
- Control of the excited state injection yield with potential determining cations and the excited state dipole orientation.
-
- Prevention of charge recombination by intramolecular electron transfer from a covalently bonded electron donor. Our most recent work in this area has been done in collaboration with Curtis Berlinguette and his coworkers at the University of British Columbia.
-
- Utilization of time resolved anisotropy measurements to quantify lateral intermolecular electron transfer reactions that occur after excited state injection.
- Quantification of the role surface electric fields play on interfacial electron and ion transfer chemistry as well as the non-Nernstian behavior of molecules at semiconductor interfaces.
Photocatalysis that stores solar energy in the form of covalent bonds in fuels is also of particular interest. In this regard, iodide oxidation continues to be the focus of much basic research as the electrons abstracted from iodide can be used to generate fuels from water or CO2. In future work, we will expand these studies to water oxidation through the UNC-EFRC. Specific emphasis will be placed on inventing new photo-chemistry where excited state electron transfer and chemical bond formation occur in one concerted step.
Professional Background
Ph.D., University of Wisconsin at Madison, 1989
B.S. State University of New York at Albany, 1985
Research Group
News & Publications
At the center's Chapel Hill headquarters, more than 100 researchers work to turn sunlight into methanol.
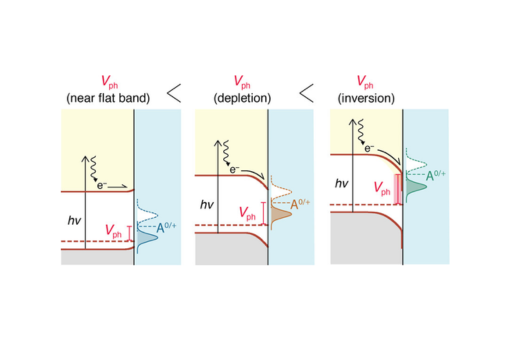
Photovoltages for hydrogen-terminated p-Si(111) in an acetonitrile electrolyte were quantified with methyl viologen [1,1′-(CH3)2-4,4′-bipyridinium](PF6)2, abbreviated MV2+, and [Ru(bpy)3](PF6)2, where bpy is 2,2′-bipyridine, that respectively undergo two and three one-electron transfer reductions.