Department News

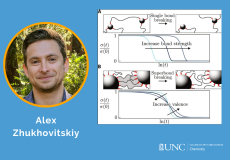
The UNC team asked whether salicylaldehydes could be used to set a molecule’s shape early on and then carry that information through later steps.

Saba Mahmoodpour is the lead author of a recent Journal of Physical Chemistry paper, which focuses on a powerful new technique called nonlinear photocurrent spectroscopy, or NLPC, which uses pairs of short laser pulses to measure how quickly electrical charges move inside thin-film solar cells.

The research revealed a strong, direct relationship between the amount of brown carbon in the smoke and how much light the particles absorb, especially at ultraviolet wavelengths.

The Department of Chemistry at the University of North Carolina at Chapel Hill is delighted to announce the promotion of two outstanding faculty members to the rank of Professor, effective January 1, 2026.
Research

Herein, an approach for discriminating between tardigrade morphological states is developed and utilized to compare sucrose- and CaCl2-induced tuns, using the model species Hypsibius exemplaris.

Herein, we disclose a backbone rearrangement approach to tune the short-chain branching of polymers.
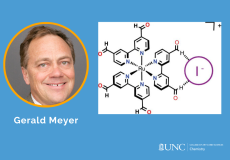
A new homoleptic Ru polypyridyl complex bearing two aldehyde groups on each bipyridine ligand, [Ru(dab)3](PF6)2, where dab is 4,4′-dicarbaldehyde-2,2′-bipyridine, was synthesized, characterized, and utilized for iodide photo-oxidation studies.
Connect