Department News

In her lab at Wake Forest University, Assistant Professor Katherine Albanese is reimagining proteins as tools for probing life at the molecular level—decoding how subtle chemical tags on DNA-packaging proteins affect gene expression and, ultimately, health.

UNC-Chapel Hill chemistry researchers have discovered a new way to attach powerful carbon dioxide-reducing molecules to silicon surfaces that could help scientists harness sunlight to turn carbon dioxide into useful fuels and chemicals.

Walk through UNC’s modern chemistry complex and you’ll see Lowry Caudill’s influence everywhere—on the facilities, the fundraising campaigns, the student experiences and, perhaps most enduringly, the values that guide the department’s mission.

From investigating untreatable diseases and sustainable energy storage to creating programs for a more connected Carolina, Rodriguez crafted a dynamic Ph.D. experience that enabled her to thrive in the halls of Kenan and in BASF’s prestigious Leadership Development Program.

The MX908, developed by UNC and 908 Devices, combines thermal desorption, chemical ionization and tandem mass spectrometry to detect hundreds of analytes at trace levels including illicit drugs.

Researchers found that certain PFAS may do more than just hang around. They might also make some breast cancers more aggressive over time.
Research

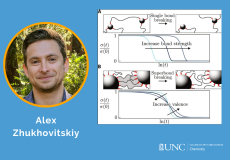
Herein, an approach for discriminating between tardigrade morphological states is developed and utilized to compare sucrose- and CaCl2-induced tuns, using the model species Hypsibius exemplaris.

Herein, we disclose a backbone rearrangement approach to tune the short-chain branching of polymers.
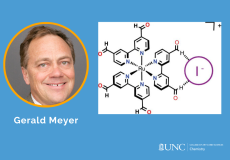
A new homoleptic Ru polypyridyl complex bearing two aldehyde groups on each bipyridine ligand, [Ru(dab)3](PF6)2, where dab is 4,4′-dicarbaldehyde-2,2′-bipyridine, was synthesized, characterized, and utilized for iodide photo-oxidation studies.