Stereoselective Polymerization of 3,6-Disubstituted N-Vinylcarbazoles
Abstract
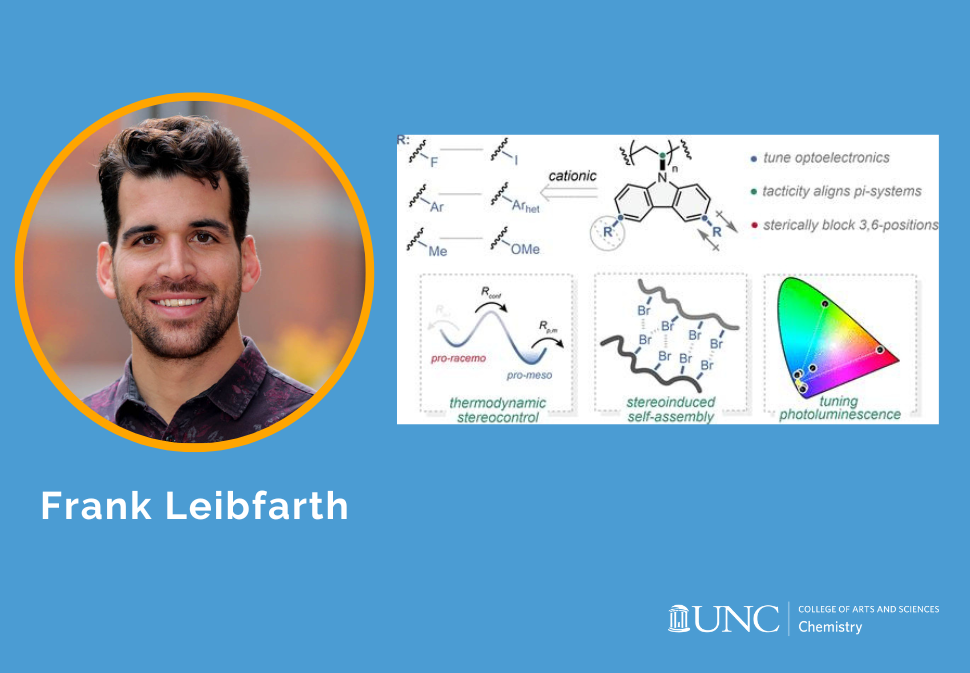
Poly(N-vinylcarbazole) (PNVC-H) is a valuable nonconjugated photoconductive polymer, but the free radical polymerization conditions typically used for its synthesis do not control polymer stereochemistry and are not tolerant to many substituted N-vinylcarbazoles. Here, we report the stereoselective cationic polymerization of a series of 3,6-disubtituted N-vinylcarbazole derivatives using a chiral scandium-bis(oxazoline) Lewis acid catalyst. The combination of asymmetric ion-pairing catalysis and inherent monomer stereoelectronics facilitated stereoselective polymerization at room temperature, which enabled the polymerization of less soluble 3,6-disubstituted-N-vinylcarbazole derivatives. Isotactic halogen-substituted PNVCs demonstrated self-assembly in solution through halogen–halogen bonding, which was not observed in their atactic counterparts. Initial spectral characterization displayed a wide range of excitation–emission profiles for substituted PNVCs, which demonstrate the promise of these materials as a new class of nonconjugated photoconductive polymers for optoelectronic applications. Overall, these results showcase a diverse class of isotactic poly(N-vinylcarbazoles), highlight the benefits of identifying alternative stereocontrol mechanisms for polymerization, and expand the suite of accessible nonconjugated hole-transport materials.
Citation
Stereoselective Polymerization of 3,6-Disubstituted N-Vinylcarbazoles
Cole C. Sorensen, Anthony Y. Bello, and Frank A. Leibfarth
ACS Macro Letters 2024 13 (5), 614-620
DOI: 10.1021/acsmacrolett.4c00191