Salt Effect on the Viscosity of Semidilute Polyelectrolyte Solutions: Sodium Polystyrenesulfonate
Abstract
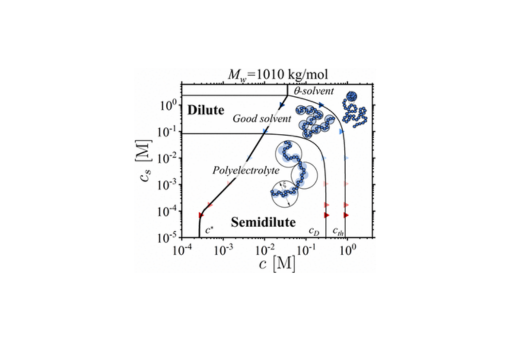
We studied the viscosity of semidilute aqueous solutions of sodium polystyrenesulfonate as a function of polymer and salt concentrations. The viscosity data were quantified by applying a scaling relationship between solution correlation length ξ = lgν/B and number of monomers g per correlation volume for chains with monomer projection length l. The specific values Bpe,Bg, and Bth of the B-parameter corresponding to exponents ν = 1, 0.588, and 0.5 were determined by the fraction of charged monomers and their degree of ionization, the effective solvent quality for the polymer backbone, the chain Kuhn length, and the type and strength of monomer–solvent interactions. The values of the B-parameters were obtained from the plateaus of normalized specific viscosity ηsp(c)/Nw(cl3)1/(3ν–1) as a function of the monomer concentration c for polyelectrolytes with weight-average degree of polymerization Nw. The extension of this approach to the entangled solution regime allowed us to determine the packing number of a chain of correlation blobs, P̃e, which completes the set of parameters {Bpe, Bg, Bth, P̃e} uniquely describing static and dynamic properties of polyelectrolyte solutions. This information was used to construct a diagram of states, calculate the fraction of free counterions and the energy of the electrostatic blobs, and establish a crossover concentration to the entangled solution regime.
Citation
Salt Effect on the Viscosity of Semidilute Polyelectrolyte Solutions: Sodium Polystyrenesulfonate
Anish Gulati, Michael Jacobs, Carlos G. Lopez, and Andrey V. Dobrynin
Macromolecules 2023 56 (5), 2183-2193
DOI: 10.1021/acs.macromol.2c02128