Mechanistic Insight into the Stereoselective Cationic Polymerization of Vinyl Ethers
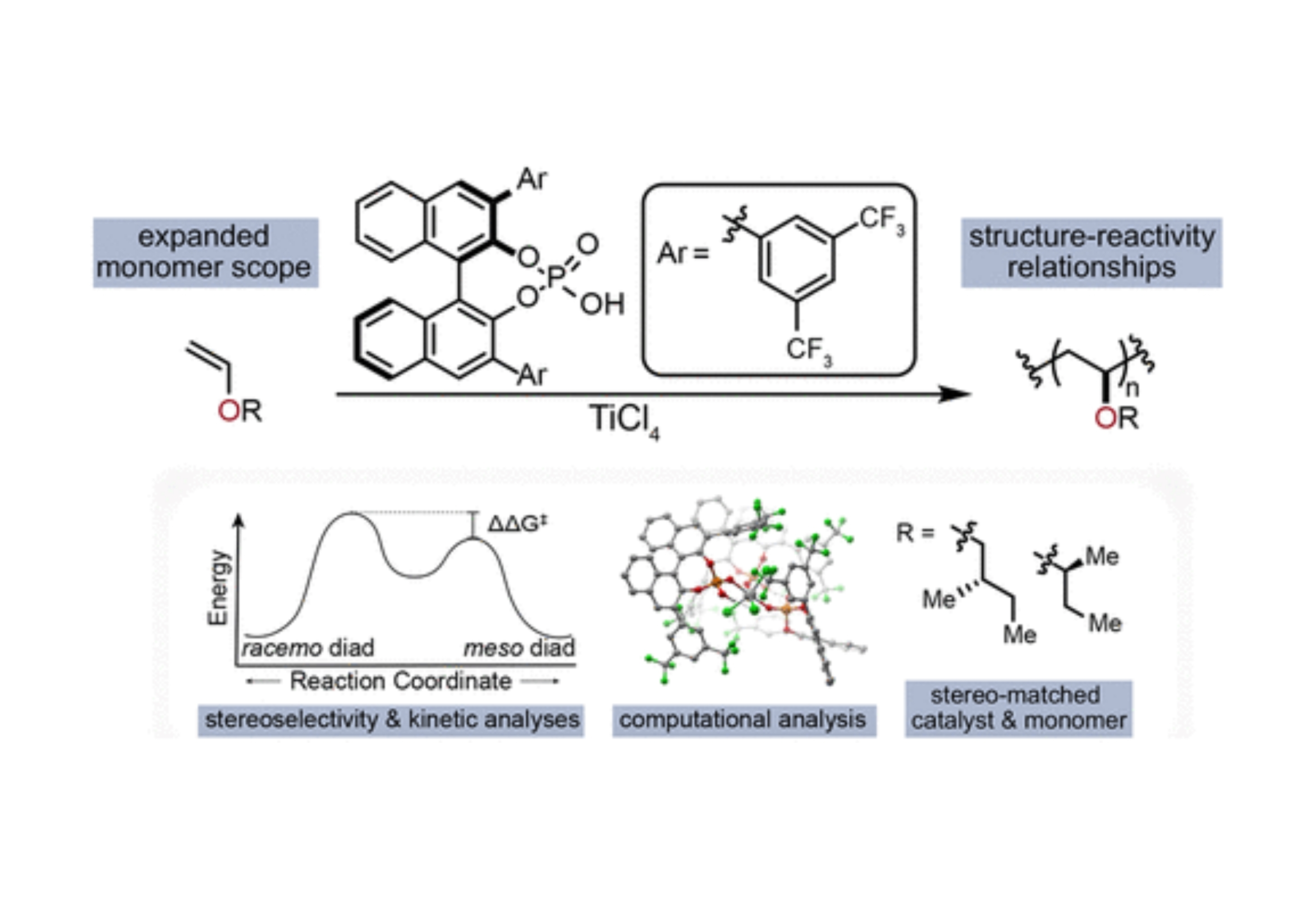
Congratulations to graduate student Travis Varner who, with the help of postdoc Aaron Teator and UNC undergraduate Paige Jacky, started a long journey three years ago to understand the mechanism of our previously published stereoselective polymerization of vinyl ethers. While our initial paper demonstrated an unprecedented method for the synthesis of highly isotactic poly(vinyl ether)s, it left many questions unanswered on the mechanism of the reaction: Why was so much ligand needed? Why was order of addition so important? How can we expand the scope to other substrates? The team performed a wide range of experimental stuides to try and answer these question, including lots of time measuring kinetics using in situ IR to understand the Arrhenius behavior of the system. An Eyring analysis of stereoselectivity provided quantitative values for the difference in transition state energies that lead to stereoselectivity. To understand the solution-state catalyst structure, the help of valuable collaborators Dr. Yernaidu Reddi and Prof. Chris Cramer from the University of Minnesota provided detailed computational information. As a fortuitous outcome of their fundamental work, the group discovered that using enantioenriched monomers led to a match-mismatch effect that resulted in the highest selectivity ever reported for a poly(vinyl ether) 95 % meso diads. To learn all the gory details, click Read More.
Citation
Mechanistic Insight into the Stereoselective Cationic Polymerization of Vinyl Ethers
Travis P. Varner, Aaron J. Teator, Yernaidu Reddi, Paige E. Jacky, Christopher J. Cramer, and Frank A. Leibfarth
Journal of the American Chemical Society 2020 142 (40), 17175-17186
DOI: 10.1021/jacs.0c08254