Heterointerface Engineering of Ni2P–Co2P Nanoframes for Efficient Water Splitting
Abstract
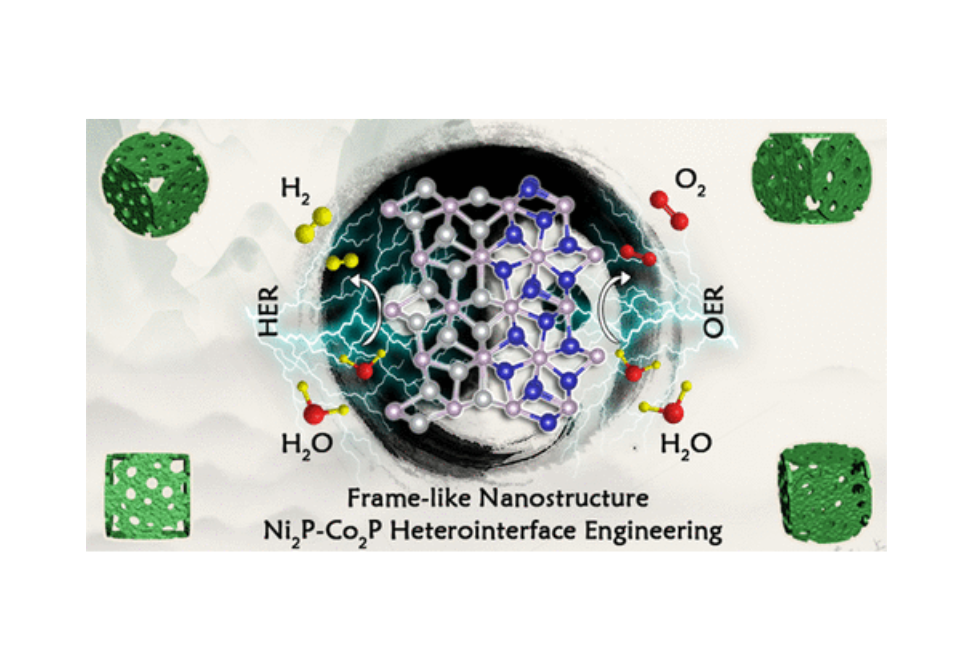
Designing highly active, stable, bifunctional, noble-metal-free electrocatalysts for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) is a major challenge in water splitting. We report here a novel, frame-like nanostructured catalyst (Ni,Co)2P nanoframe (NF), which consists of heterostructured Ni2P–Co2P nanoparticles embedded in the N-doped carbon matrix. Its synthesis involves precipitation, chemical etching, and a final phosphidation step to give an optimized electronic structure that contains multiple catalytic active sites and facilitates mass transfer. The catalyst is a bifunctional catalyst for both HER and OER and is superior to the individual components Ni2P and Co2P samples and (Ni,Co)2P solid nanocubes. When the (Ni,Co)2P NF catalyst was employed as both the cathode and anode for overall water splitting, a remarkably low cell voltage of 1.54 V was required to achieve a current density of 10 mA cm–2. Density functional theory calculations verify the strong electronic interaction between Ni2P and Co2P at the heterointerfaces, resulting in an optimized hydrogen adsorption strength for enhanced HER electrocatalysis. Moreover, the synthetic strategy has been generalized for the synthesis of Ni–Co dichalcogenide NFs, thereby holding a great promise for a variety of potential applications.
Citation
Heterointerface Engineering of Ni2P–Co2P Nanoframes for Efficient Water Splitting
DOI: 10.1021/acs.chemmater.1c02609