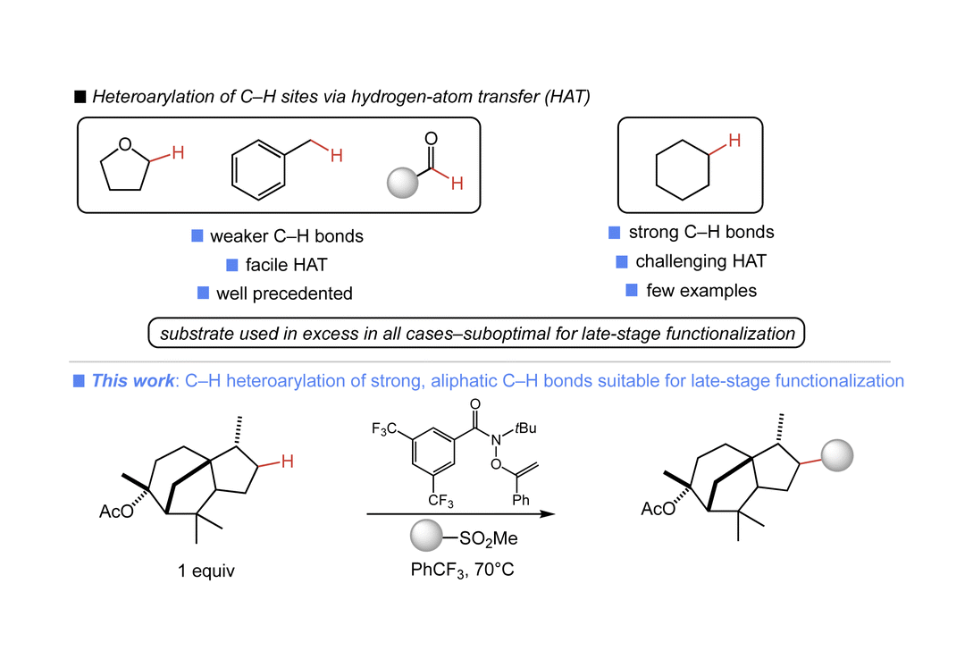
Heteroarylation of unactivated C–H bonds suitable for late-stage functionalization
Abstract
The late-stage introduction of diverse heterocycles onto complex small molecules enables efficient access to new medicinally relevant compounds. An attractive approach to such a transformation would utilize the ubiquitous aliphatic C–H bonds of a complex substrate. Herein, we report a system that enables direct C–H heteroarylation using a stable, commercially available O-alkenylhydroxamate with heterocyclic sulfone partners. The C–H heteroarylation proceeds efficiently with a range of aliphatic substrates and common heterocycles, and is a rare example of heteroarylation of strong C–H bonds. Importantly, the present approach is amenable to late-stage functionalization as the substrate is the limiting reagent in all cases.
Citation
Heteroarylation of unactivated C–H bonds suitable for late-stage functionalization. Miller, Austin S., Alexanian, Erik J. 2022. Chemical Science. The Royal Society of Chemistry. http://.doi.org/10.1039/D2SC04605A.