Diastereoselective Synthesis of the ABCD Ring System of Rubriflordilactone B
Abstract
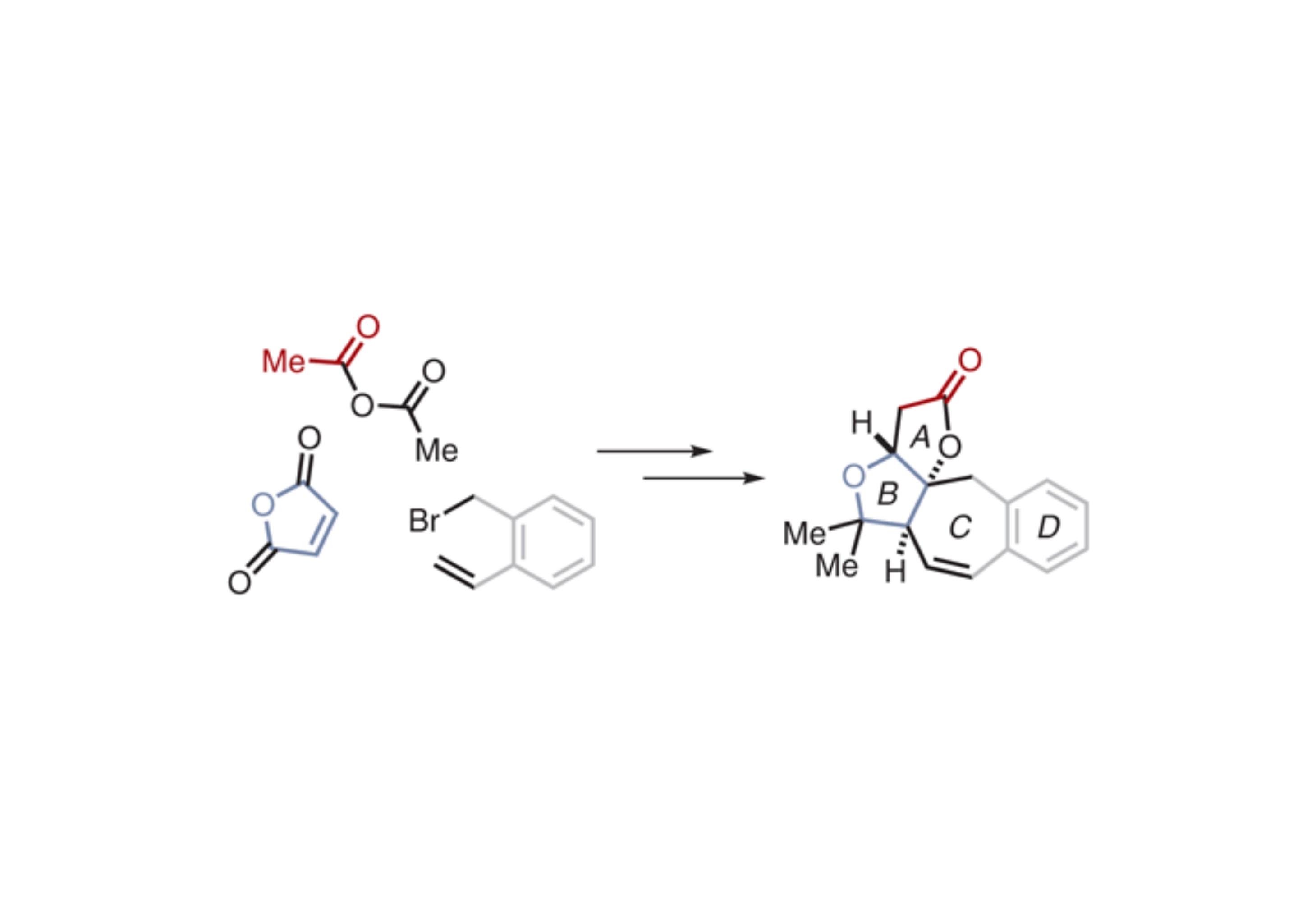
A novel nine-step diastereoselective route to the ABCD ring system of the natural product rubriflordilactone B is reported. Use of an alpha-substituted butenolide derived from maleic anhydride facilitated a 1,4-conjugate addition to provide a diene. The order in which a ring-closing metathesis and enolate oxidation were performed on this compound dictated the relative stereochemistry of the target. The final product exhibited anisotropic effects during room-temperature NMR studies, requiring elevated-temperature experiments to confirm its identity.
Citation
Diastereoselective Synthesis of the ABCD Ring System of Rubriflordilactone B
Hudson G. Roth and David A. Nicewicz. Synlett. DOI: 10.1055/a-1659-6521