Determining the Overpotential of Electrochemical Fuel Synthesis Mediated by Molecular Catalysts: Recommended Practices, Standard Reduction Potentials, and Challenges
Abstract
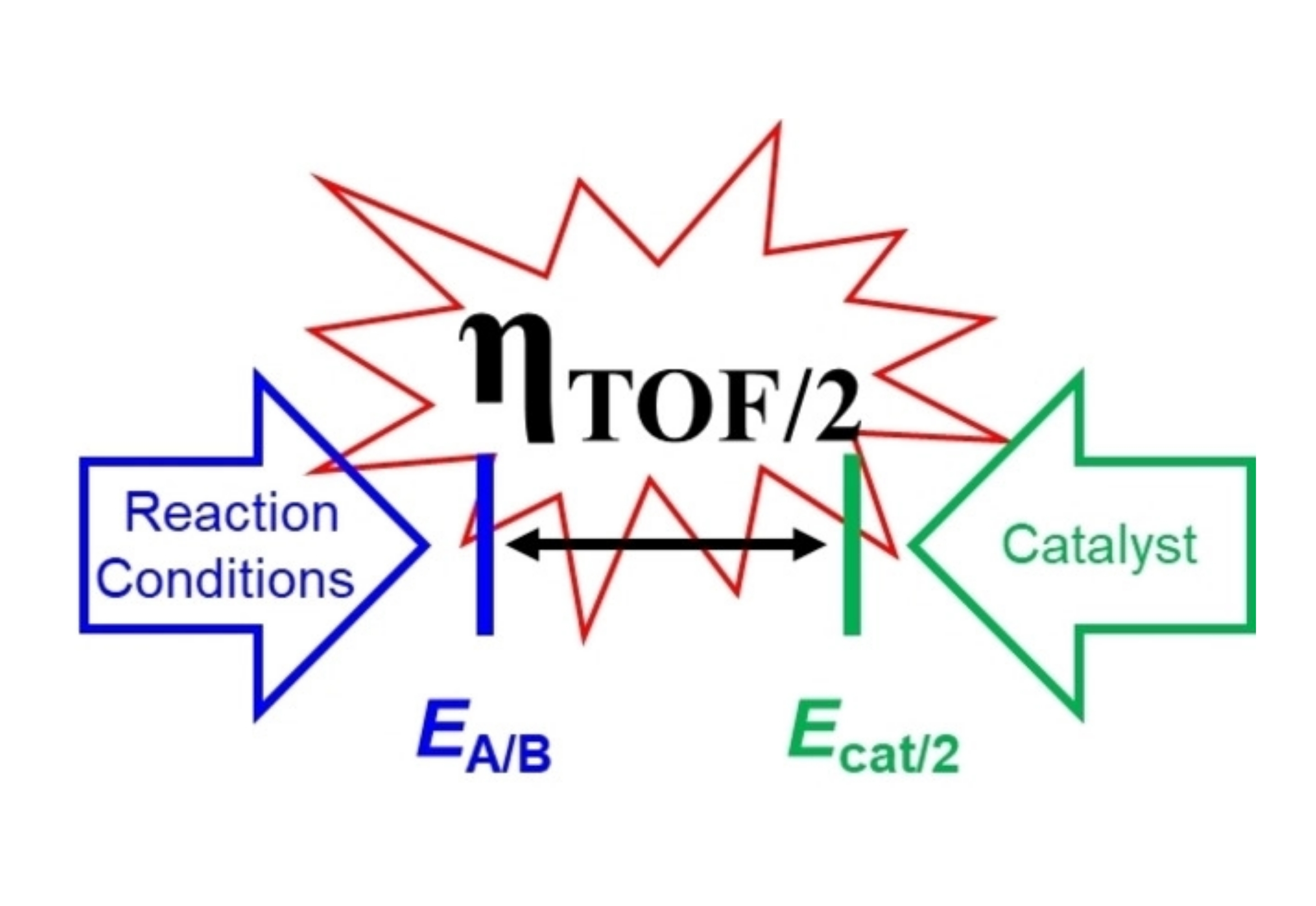
Molecular catalysts capable of performing electrochemical transformations are frequently utilized in the synthesis of fuels. A common benchmark for evaluating catalysts for electrochemical reactions is overpotential (η) and minimizing η remains an active goal in catalysis. This Review details approaches for the determination of thermodynamic potentials for common fuel-forming reactions and for the determination of electrochemical overpotential, and underscores the need to employ methods that enable meaningful comparisons between molecular catalysts. Strategies for minimizing overpotential while maintaining high activity are highlighted.
Citation
Determining the Overpotential of Electrochemical Fuel Synthesis Mediated by Molecular Catalysts: Recommended Practices, Standard Reduction Potentials, and Challenges
Bethany M. Stratakes,Prof. Jillian L. Dempsey, and Prof. Alexander J. M. Miller
DOI: https://doi.org/10.1002/celc.202100576