Catalytic, Asymmetric Michael-Aldol Annulations via a Stereodivergent and Stereoconvergent Path Operating under Curtin-Hammett Control
Abstract
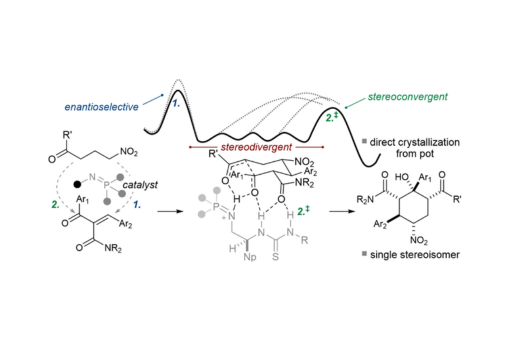
A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters (diastereoselection up to >20:1, enantioselectivity up to >99:1) in a Michael/aldol domino reaction between trisubstituted electrophilic alkenes and γ-nitroketones. Mechanistic studies suggest a scenario in which stereoconvergency is achieved by kinetically controlled cyclization after the initial diastereodivergent Michael addition. Diastereoconvergency during cyclization is shown to result from Curtin–Hammett kinetics, a finding that contrasts the crystallization-driven stereoconvergency previously reported in similar systems. Despite the change in the stereocontrol mechanism, the operational attributes remain attractive, with the crystalline products typically isolated in analytically pure form upon filtration of the reaction mixture.
Citation
Catalytic, Asymmetric Michael-Aldol Annulations via a Stereodivergent/Stereoconvergent Path Operating under Curtin–Hammett Control
Mitchell T. Giordano, Katelyn M. Kitzinger, Pedro de Jesús Cruz, Shubin Liu, and Jeffrey S. Johnson
Journal of the American Chemical Society 2023 145 (22), 12370-12376
DOI: 10.1021/jacs.3c03373