March 8, 2023 | By the Kanai Group
This week’s issue of Physical Review Letters (PRL) features the article “Electronic Excitation Response of DNA to High-energy Proton Radiation in Water” by Chris Shepard, Dr. Dillon Yost, and Professor Yosuke Kanai. The article was chosen and highlighted as PRL Editor’s Suggestion.
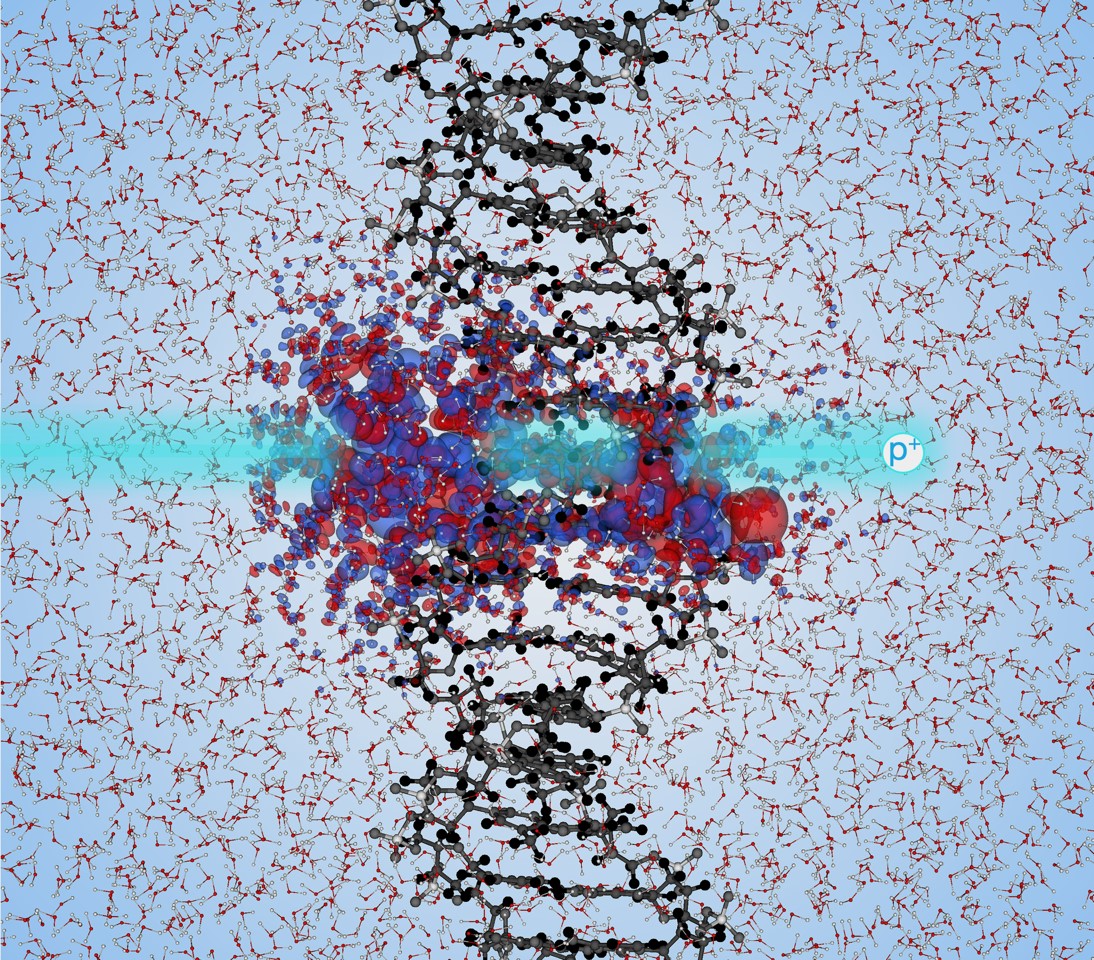
Understanding the radiation-induced response of DNA is pivotal for human health. The electronic excitation induced in DNA by high-energy protons is central to understanding how DNA damage occurs in extreme conditions such as those experienced by astronauts. For instance, as much as 90% of galactic cosmic radiation (GCR) is high-energy protons, and human exposure to GCR is a great concern for space missions. The electronic response of DNA to high-energy protons is also the foundation of modern proton beam cancer therapy. The energy transfer rate from irradiating protons to electrons in the target matter is quantified by so-called electronic stopping power, and it plays a central role in understanding the electronic stopping phenomenon. Starting with the seminal work by Hans Bethe in 1930, many researchers have developed linear-response models for calculating the electronic stopping power. Over the last ten years, Prof. Kanai’s group has developed a new computational formalism such that quantum dynamics responsible for electronic stopping is directly simulated from first-principles quantum mechanical theory[1]. Building on their earlier work on water[2], the new PRL discusses how sugar-phosphate side chains of DNA respond strongly to irradiating protons due to the lone-pair electrons when DNA is solvated in water. As a result, positively charged holes are formed with a greater probability on the DNA side chains than on the nucleobases. This work advances our understanding of how the exposure of DNA to highly energetic protons can result in double-strand breaks in DNA, which are particularly important in inducing cell death.