
Jeffrey Johnson
A. Ronald Gallant Distinguished Professor
Caudill Laboratories 220919-843-4936
jsj25@email.unc.edu
Group Website
Curriculum Vitae
Research Interests
Synthetic Organic Chemistry, Asymmetric Catalysis
Research Synopsis
My interests lie in the general area of chemical synthesis. I am particularly interested in the discovery of new organic transformations and their application to the total synthesis of architecturally challenging and biologically important natural products. Our criteria for the development of a synthetic method may be summarized by three characteristics:
One
The reaction should achieve several structural modifications in a single synthetic operation: tandem or domino reactions are optimal;
Two
The reaction should introduce a significant level of stereochemical complexity in a controlled fashion and absolute stereocontrol should arise from a chiral source used in catalytic quantities;
Three
The starting materials should be readily accessed, but be easily transformed into complex products. To meet these challenges, students in my group will learn a full complement of organic and organometallic chemistry.
Professional Background
B.S., University of Kansas, 1994; Ph.D., Harvard University, 1999; NIH Postdoctoral Fellow, University of California, Berkeley, 1999-2001; Research Corporation Research Innovation Award, 2002; National Science Foundation CAREER Award, 2003-2008; 3M Nontenured Faculty Award, 2003; UNC Junior Faculty Development Award, 2004; Johnson and Johnson Focused Giving Award, 2004-2006; Eli Lilly Grantee, 2004; Amgen Young Investigator Award, 2005; GSK Scholar Award, 2006-2007; Alfred P. Sloan Fellow, 2006-2008; Camille Dreyfus Teacher Scholar, 2006-2009; Ruth and Phillip Hettleman Prize for Artistic and Scholarly Achievement, 2006; Novartis Early Career Award in Organic Chemistry, 2008; American Chemical Society's Arthur C. Cope Scholar Award in Recognition of Excellence in Organic Chemistry, 2010; Elias J. Corey Award, 2012; Society of Synthetic Organic Chemistry, Japan Lectureship Award, 2014; Japanese Society for the Promotion of Science Fellow, 2015; William C. Friday, Class of 1986, Award for Excellence in Teaching, 2016, Journal of Organic Chemistry Outstanding Author of the Year 2016, AAAS Fellow 2019
Research Group
News & Publications

Chemists in the Johnson Lab convert chemicals from petroleum waste into compounds for study as anesthetics and antidepressants.
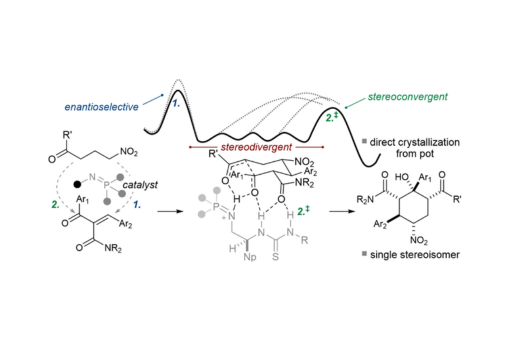
A bifunctional iminophosphorane (BIMP)-catalyzed method for the synthesis of densely functionalized cyclohexanols establishes five contiguous stereocenters.


