Polymer Skeletal Editing via Anionic Brook Rearrangements
Abstract
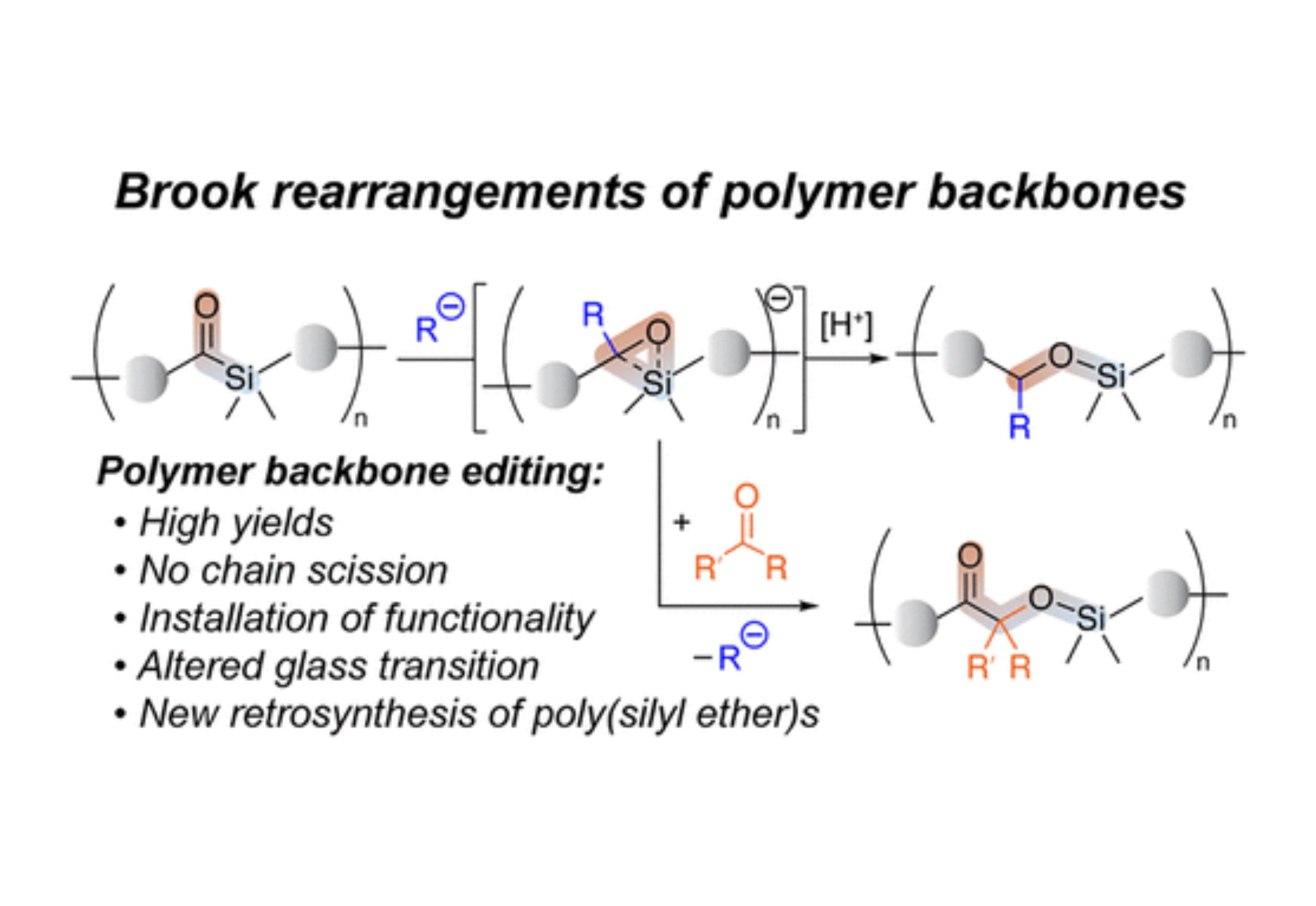
This report communicates the first example of polymer backbone metamorphosis affected by anionic 1,2-Brook rearrangement of acyl silane moieties. Introduction of the acyl silane functionality into a polymer backbone was achieved via acyclic diene metathesis copolymerization (ADMET) of diene 1 and two dienes. We demonstrate that, using organolithium species and cyanide as nucleophiles, the backbones of resulting copolymers can be triggered to undergo highly efficient 1,2-Brook rearrangement, which transforms the poly(acyl silane)s into poly(silyl ether)s. Furthermore, the carbanion intermediate of the 1,2-Brook rearrangement can be intercepted by ketone electrophiles to give rise to polymers with quaternary stereogenic centers in the backbone and pendant functionality. Such structural editing of polymer backbones enables a new retrosynthetic paradigm for silicon-containing polymers that could not be accessed by traditional means.
Citation
Polymer Skeletal Editing via Anionic Brook Rearrangements
Maxim Ratushnyy and Aleksandr V. Zhukhovitskiy
Journal of the American Chemical Society 2021 143 (43), 17931-17936
DOI: 10.1021/jacs.1c06860