Accessing Photoredox Transformations with an Iron(III) Photosensitizer and Green Light
Abstract
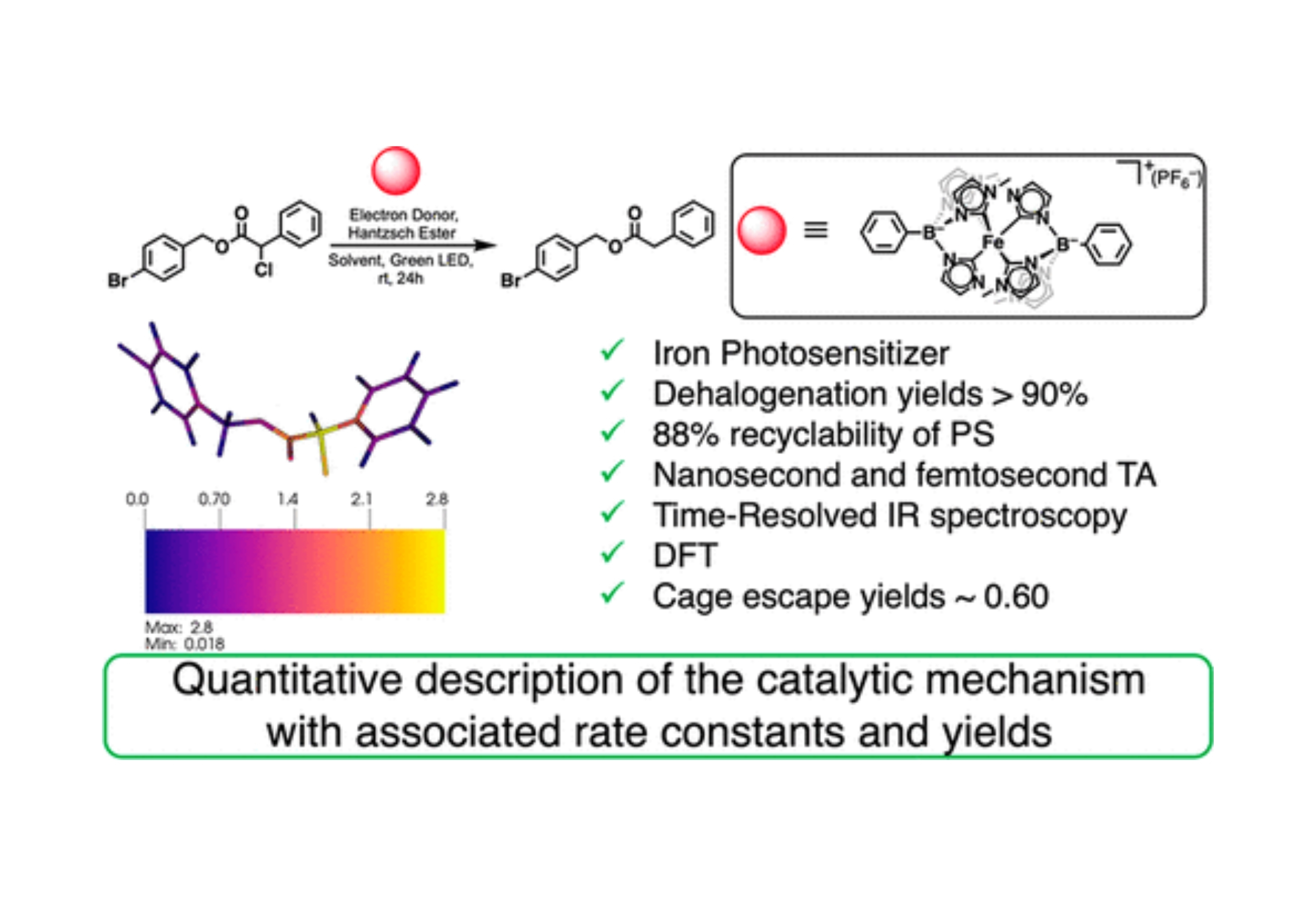
Efficient excited-state electron transfer between an iron(III) photosensitizer and organic electron donors was realized with green light irradiation. This advance was enabled by the use of the previously reported iron photosensitizer, [Fe(phtmeimb)2]+ (phtmeimb = {phenyl[tris(3-methyl-imidazolin-2-ylidene)]borate}, that exhibited long-lived and luminescent ligand-to-metal charge-transfer (LMCT) excited states. A benchmark dehalogenation reaction was investigated with yields that exceed 90% and an enhanced stability relative to the prototypical photosensitizer [Ru(bpy)3]2+. The initial catalytic step is electron transfer from an amine to the photoexcited iron sensitizer, which is shown to occur with a large cage-escape yield. For LMCT excited states, this reductive electron transfer is vectorial and may be a general advantage of Fe(III) photosensitizers. In-depth time-resolved spectroscopic methods, including transient absorption characterization from the ultraviolet to the infrared regions, provided a quantitative description of the catalytic mechanism with associated rate constants and yields.
Citation
Accessing Photoredox Transformations with an Iron(III) Photosensitizer and Green Light
Akin Aydogan, Rachel E. Bangle, Alejandro Cadranel, Michael D. Turlington, Daniel T. Conroy, Emilie Cauët, Michael L. Singleton, Gerald J. Meyer, Renato N. Sampaio, Benjamin Elias, and Ludovic Troian-Gautier
Journal of the American Chemical Society 2021 143 (38), 15661-15673
DOI: 10.1021/jacs.1c06081