
Michel Gagné
Mary Ann Smith Distinguished Professor of Chemistry
Caudill Laboratories 218919-962-6341
mgagne@unc.edu
Group Website
Curriculum Vitae
Research Interests
Catalysis, Synthetic Methods, Synthetic Receptors, Biofuels
Research Synopsis
Research in the Gagné group focuses on catalysis and synthetic methods. We develop catalytic methods for carrying out selective transformations in complex environments under catalyst, such as kinetic, control. We seek to control where a catalyst reacts and what reaction/s it promotes in multi-functional environments to modify complex structures for many applications; we are currently concentrating on biomass conversion and the late-stage functionalization of bioactive molecules. We also have interests in de novo design of peptide-based catalysts to create artificial enzymes, and new organometallic silyl complexes.
Biomass Conversion
Here we seek to convert biorenewable carbon sources such as cellulose into high-value chemicals, which are currently accessed from petroleum roots. Since the most valuable chemicals are partially oxidized, they are accessed by the oxidation of petroleum resources. On the other hand, biomass is an over-oxidized source of carbon, each carbon in glucose is at the alcohol or aldehyde oxidation state, and so the challenge is to develop chemistries for reducing, or more precisely, deoxygenating biomass.
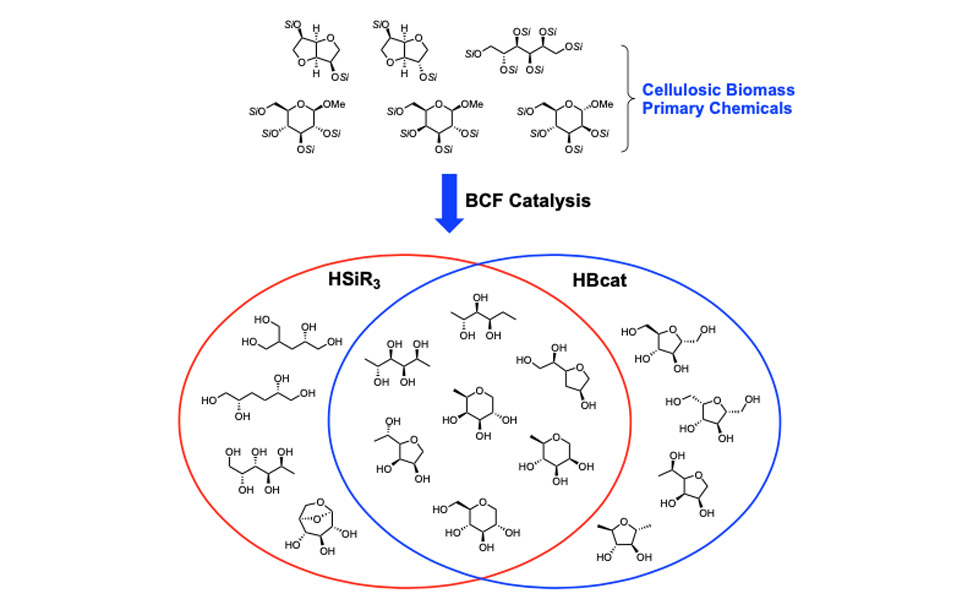
To achieve this goal in a site-selective manner, we have turned to non-transition metal catalysts for the activation of silanes. The fluoroaryl borane catalyst B(C6F5)3 is unique in its ability to heterolytically activate silanes, and we have used it to develop strategies for the site-selective deoxygenation of cellulosic biomass. We have also discovered that hydro-boranes can be activated in the same way as hydro-silanes for deoxygenation chemistry. The following Venn diagram shows the compounds we have directly synthesized from a primary biorenewable, glucose, sorbitol, isosorbide, et cetera. Silanes and boranes exhibit unique selectivities.
Late-Stage Functionalization
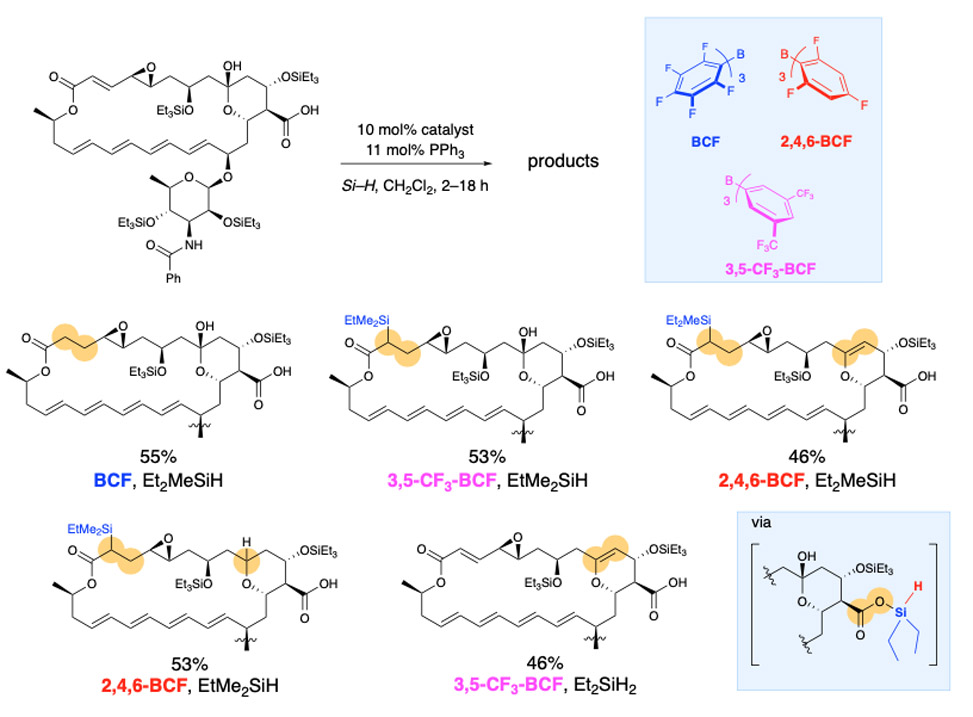
The strategies we have developed for achieving site-selectivity in poly-ols, also serves us well in our goals of site- and chemo-selective reactivity in poly-functional bioactive natural products. Here the challenges are magnified as there are many more potentially reactive functional groups. We seek catalyst control over site-selectivity in complex natural products as it enables late stage functionalization, which provides structurally modified compounds for structure activity relationship, SAR, studies, optimization of biological efficacy, bioavailability, et cetera. In the example described below the anti-fungal natamycin is modified at different sites depending on which catalyst is used.
Professional Background
Ph.D., Northwestern University, 1991; NSERC of Canada Postdoc at Caltech, 1991-1992, and Harvard University, 1992-1995; CaRLa Fellow, University of Heidelberg, 2008; Exeter College Visiting Fellow, Oxford University, 2006; SCR Member Wadham College, Oxford University, 2005-2006; W. R. Kenan Jr. Leave, 2005-2006; NSERC GSC-24 Grants Selection Committee, 2005-2008; Canadian Journal of Chemistry Board of Editors, 2002-2005; Camille-Dreyfus Teacher Scholar Award, 2000; Union Carbide Innovation Recognition Award, 2000; 3M Untenured Faculty Award, 1999; NSF Career Award, 1996; Fellow, American Association for the Advancement of Science, 2012; Royal Society of Chemistry, “Catalysis in Organic synthesis” Award, 2016; Fellow, Royal Society of Chemistry, 2016.
News & Publications

In an effort to redesign the Chemistry Department’s Course-based Undergraduate Research Experience (CURE), Dr. Jade Fostvedt, in collaboration with the Chemistry Department’s Dr. Michel Gagné, Dr. Jillian Dempsey, and Dr. Kathleen Nevins, set out to create a new project for CHEM 550L, a capstone synthetic chemistry laboratory course, and the proposed course was one of the recipients of the UNC Systems Research Program Award.
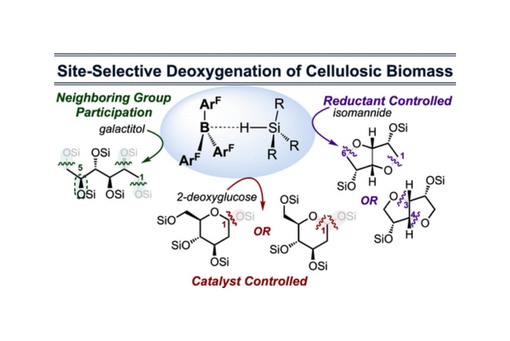
This perspective describes key lessons from a program whose goals are to site-selectively deoxygenate cellulose-derived carbohydrates.

