Using NMR-detected hydrogen-deuterium exchange to quantify protein stability in cosolutes, under crowded conditions in vitro and in cells
Abstract
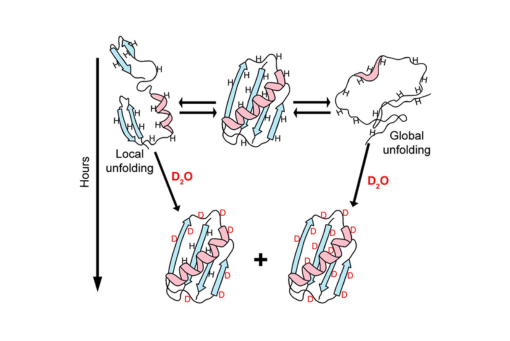
We review the use of nuclear magnetic resonance (NMR) spectroscopy to assess the exchange of amide protons for deuterons (HDX) in efforts to understand how high concentration of cosolutes, especially macromolecules, affect the equilibrium thermodynamics of protein stability. HDX NMR is the only method that can routinely provide such data at the level of individual amino acids. We begin by discussing the properties of the protein systems required to yield equilibrium thermodynamic data and then review publications using osmolytes, sugars, denaturants, synthetic polymers, proteins, cytoplasm and in cells.
Citation
Chu, I.-T., & Pielak, G. J. (2023). Using NMR-detected hydrogen-deuterium exchange to quantify protein stability in cosolutes, under crowded conditions in vitro and in cells. Magnetic Resonance Letters. https://doi.org/10.1016/j.mrl.2023.04.003